International Journal of Agricultural Science and Food Technology
Engineering the plant genome, transient gene silencing, meristematic gene expression and up regulation of flowering - The phase change in papaver bracteatum
Phani Raja Kumar Madam*
Cite this as
Kumar Madam PR (2020) Engineering the plant genome, transient gene silencing, meristematic gene expression and up regulation of flowering - The phase change in papaver bracteatum. Int J Agric Sc Food Technol 6(2): 157-165. DOI: 10.17352/2455-815X.000067CRISPR - Cas9 for gene editing has long been considered revolutionary in minimizing time frame to improve plant genetics and crop breeding. By using CRISPR tools we can improve desired traits, such as yield, plant height, gene expression, gene silencing, and disease tolerance. Flowering in plants is regulated by complex network of gene-controlled factors. This paper particularly aims at CRISPR-induced double-strand breaks used to create a gene “knock-ins” by exploiting the cells’ homology-directed repair. The precise insertion of a donor template can alter the coding region of a gene. Altering the Cas9 protein so it cannot cut DNA, transient gene silencing or transcriptional repression can also be done. The modified Cas9, led by a guide RNA, targets the promoter region of a gene and reduces transcriptional activity and gene expression. CRISPR/Cas9 genome-editing system based on RNA endoribonuclease to induce high-efficiency and inheritable targeted deletion of transcription factors involved in floral development in Papaver bracteatum. By using AP1, SVP, and TFL1 as the target genes, multisite and multiple-gene mutations were achieved to express multiplexed sgRNAs from a single transcript driven by the promoter in transgenic lines. Targeted deletions of chromosomal fragments between the first exon and second exon in either one or three genes were generated by using a single binary vector. Interestingly, the efficiency of site-targeted deletion was comparable to that of mutation with the multiplexed sgRNAs. DNA sequencing analysis of RT-PCR products showed that targeted deletions of AP1 and TFL1 may lead to frameshift mutations of the target genes. In addition, no RT-PCR amplified product was acquired after SVP targeted deletion. Furthermore, the targeted deletions resulted in abnormal floral development in the mutant lines compared to that of un-treated plants. AP1 and SVP mutations increased plant branching expressively, while treated mutant plants displayed a change from indeterminate to determinate inflorescences. Results demonstrate that CRISPR/Cas9 with the RNA endoribonuclease Csy4 processing system is an efficient tool to study floral development and improve floral traits rapidly and phase change from Juvenility to flowering phase was tracked using shoot apical meristematic genes by vernalization and other gene editing factors.
Introduction
Juvenility, or ripeness to flower, has been defined as the condition of the plant before it is mature enough to flower under normally inductive conditions (Salisbury and Ross, 1985). Higher plants are unable to initiate flowering immediately after germination and must undergo a process of maturation, or juvenile developmental phase [1]. This transition from juvenile to adult characteristics is termed as phase change [2]. This phase of development in which the plant is insensitive to inductive conditions is most common with many seed- raised species [3]. Most perennial plants must pass through a significant juvenile phase of vegetative development before they are able to flower [4]. Before a plant can flower in response to environmental stimuli such as day length and vernalization (cold temperature), the organs that detect the environmental change, usually leaves or meristem, must reach a condition called “ripeness to respond” and the meristem must also be capable of responding to signals from these organs to initiate the changes leading to reproductive development. There is a great diversity among species and plant organs in the age at which they achieve this condition.
The juvenile stage can last from a few days to several weeks or years depending on species or cultivar [5]. The juvenile phase in many woody plants can be very lengthy, with Hackett [6] reporting a juvenile period of 30-40 years in some forest species while Rugini (1986) and Bellini (1993) reported a juvenile period of greater than 15 years in Olea europaea (olives). Brown (1992) reported that juvenile like phase is independent of chronological age but lasted until plants reached a minimum size or stage of development in Tanacetum cinerariaefolium L. (pyrethrum). In Heuchera species (Bressingham Hybrids), a juvenility requirement of 10 weeks must be met before satisfying a vernalization requirement for flowering [7].
Collinson, et al. (1992) reported a significant difference in the duration of juvenile phase in Oryza sativa (rice) compared to P. somniferum. Cooler temperatures (28/20ºC) prolonged the duration of juvenile phase in the four rice cultivars tested in the glasshouse conditions (Collinson, et al. 1992). Also, in Glycine max (soya bean) another short day plant, the duration of the juvenile phase was also temperature dependent unlike P. somiferum (Hodges and French, 1985; Jones and Laing, 1978). No published information exists on the length of the juvenile phase in perennial poppy species, but planting of ornamental perennial poppies is recommended for autumn if flowering is to occur in the following summer [8] so it may be assumed that a juvenile period of several weeks must be met prior to inductive environmental requirements (vernalization) being met in winter and spring for flowering to occur. Time of planting studies in perennial crops such as pyrethrum (Fulton, 1998) sown from seed demonstrate the need to complete both a juvenile and a flower induction phase if flowering is to be achieved in the same year. Identification of the length of the juvenile phase is thus important in development of management practices for perennial crops, while an understanding of the physiological basis of juvenility may lead to development of strategies to manipulate the response.
Phase change is a complex process involving environmental, hormonal and genetic factors [9]. From a research perspective, it is important to document the phase change across species to characterize the traits unique to each phase and thus gain greater understanding of the regulation of phase change [10]. Phase change indicators differ between species and may at best be a tool for approximating the timing of the change as they do not measure the underlying changes in gene expression and physiological processes that control the transition.
The duration and characteristics of the juvenile and mature phases as well as the two components of the mature phase, the vegetative phase in which the plant is competent to flower but has not received the inductive signal and the reproductive development phase [11], are unique to each plant species. No attempt has been made to establish the duration of these three major phases during the vegetative and reproductive development of P. bracteatum.
Leaf number is one of the most widely documented phase transition indicators and has been used as an indicator of the end of the juvenile phase in several plant species. Brewster [12] proposed leaf number was a stable marker of the end of juvenility in onions grown under different light conditions, whereas time, leaf area and leaf dry weigh were not. In these studies, leaf number at phase change was unaffected by varying conditions of irradiance and photoperiod during the juvenile phase. The end of juvenility in Oryza sativa (rice) has been characterized by counting either the number of days after sowing or leaf number. Most rice cultivars investigated produced a minimum of five leaves before their juvenile phase was completed [13]. In Saccharum officinarum (Sugar cane) two to three nodes are required for a positive photo-inductive response (Mangelsdorf, 1956). Williams [14] found that node number was the potential indicator to determine the response to inductive treatment in Rubus niveus (Raspberry).
Changes in leaf morphology have been used as phase change indicators in some species. At phase transition, plants of some species display very distinct changes in leaf shape or anatomy, whereas other species show more subtle and gradual transition between juvenile and adult phases [15-18]. Poethig [19]documented that shape of the leaf was one of the possible indicators of vegetative phase change in maize and many legumes with hypogeal germination. Triticum vulgare (Maize) has been one of the best examples for evaluating phase change because its leaf anatomy changes as it progresses from a juvenile to adult phase (Kerstetter and Poethig, 1998). In a study of Maize, Bluegrass, and Rice, three distantly related and physiologically distinct grass species, leaf shape rather than leaf surface anatomical features was found to be the most uniform phase change indicator [10]. The presence of leaf abaxial trichomes has been documented as an indicator of the phase change in Arabadopsis (Telfer and Poethig, 1998).
The length of the juvenile phase is very important for scheduling crop production, and in determining the time needed to produce new cultivars in breeding programs. In commercial floriculture, it is very important to predict the length of the juvenile phase to predict the accuracy of flowering times [20] for year-round flower production. Knowledge on the length of juvenile phase helps to reduce the cost and time normally required to initiate flowers. Previous studies have reported that by predicting the length of juvenile phase, the timing of inductive treatments can be optimized. For example, in Oryza sativa cv. Zuiho [21] a single inductive cycle and in chrysanthemum a period of eight consecutive short days were required to be enough for flower initiation [22]. Hence the information on the length of juvenile phase is valuable for commercial crops where there is greater potential to manipulate the growing environment and in producing uniform flowering in turn maximizing crop yield.
Materials and methods
Plant material
Genetically uniform P. bracteatum seed, collected from a single plant grown under glasshouse conditions, was used in the juvenility experiments. Plants were raised from graded seed germinated in trays filled with a moistened mix of 50% perlite, 25% coarse sand and 25% peat, a potting media found to be ideal for small seeded species (Gracie, et al. 2003). The trays were kept in controlled environmental conditions at a daily temperature of 20 ± 3˚C. The date of emergence was defined as the day when the two cotyledons had unfolded. Seedling emergence occurred approximately 2.5 weeks after sowing. Seedlings were transplanted into 12 cm diameter pots containing potting mix four weeks after sowing, when they could be handled without damage. The potting mix used for seedling growth consisted of peat, sand and pine bark (1:2:7) and had a pH of 6.0. The genome-editing vector set uses Gibson assembly cloning to create vectors for a diverse range of genome editing applications. There are three sets of modular cloning fragments. Fragment A contains the CmYLCV promoter and was amplified by the primers CmYLCV-F/ CmYLCV-RA. Fragment B contains multiple sgRNAs targeting AP1 sites and was produced by annealing the oligos gRNA1-F/gRNA1-R or gRNA2-F/gRNA-R separately. Fragment C contains the backbone of pDIRECT-21 digested by SapI restriction enzyme. These fragments were assembled using Gibson assembly cloning kits (NEB, USA) to construct pDIR21-AP1. Using the same method, we acquired pDIR21-SVP by CmYLCV-F/ CmYLCV-RS gRNA3-F/gRNA3-R and gRNA4-F/gRNA-R and pDIR21- TFL1 by CmYLCV-F/ CmYLCV-RT, gRNA5-F/gRNA5-R, and gRNA6-F/gRNA-R separately. For pDIR21-Triple, six sgRNAs were assembled by gRNA1-F/gRNA-triple-R1, gRNA3-F/gRNA-triple-R2, gRNA5-F/gRNA-triple-R3, gRNA2-F/gRNA-triple-R4, gRNA4-F/gRNA-triple-R5, and gRNA6-F/gRNA-R separately.
Genomic DNA extraction and PCR analysis Genomic DNA was extracted from Arabidopsis leaves using the CTAB method following the published protocol48. To detect mutagenesis at the desired sites, the target regions were amplified with specific primers (see supplementary information for primer sequences) using Premix Taq DNA Polymerase (Takara, Japan) with the following protocol: 94 °C for 10 min (94 °C for 30 s, 60–48 °C for 30 s, 72 °C for 2 min) for 13 cycles with touchdown −1 °C in each cycle (94 °C for 30 s, 52 °C for 30 s, 72 °C for 2 min) for 25 cycles, 72 °C for 10 min, 4 °C to hold. The PCR product was separated in a 1% agarose gel and stained with Stainer to detect chromosomal fragment deletions. Selected PCR products were cloned into the pGEM-T Easy Vector (Promega, USA) for Sanger DNA sequencing. RNA extraction and RT-PCR Total RNA was extracted using the Nucleo-Spin RNA Plant Kit (Takara), treated with DNase before use as the template for RT-PCR, analyzed in a 1.2% agarose gel and stained with standard Stainer to assess the extracted total RNA concentration and integrity. Additionally, no degradation was found in the RNA extracts, as 18S:28S was equal to 1:2 for all samples. cDNA was then generated by reverse transcription from 1 µg of total RNA using 25 U of AMV reverse transcriptase, 100 mM dNTPs, 25 U of RNase inhibitor and 100 µm Oligo-d Primers (AMV Reverse Transcriptase Kit, Promega, USA) in a 25 µl reaction volume. The reverse transcription reaction was carried out in three steps: 120 min at 36 °C, 60 min at 48 °C and 30 min at 60 °C. PCRs were performed with 1 µl of cDNA using Premix Taq DNA Polymerase (Takara, Japan). The PCR products were separated in a 1% agar agar gel and stained with to detect the cDNA of the target gene. Designated PCR products were cloned into pGEM-T Easy Vector (Promega, USA) for DNA sequencing.
The fertilizer composition in the potting mixture was as follows: slow release (5-6 months) Osmocote® granules (330g/50L), dolomite lime (330g/50L), iron sulphate (25g/50L) and trace elements (Micromax® 20g/50L). The pots were watered daily. Identical fertilizer and irrigation schedules were followed for each of the treatments. Seedlings were grown under glasshouse conditions with ambient light and a temperature of 20 ± 3˚C for 12 weeks until the imposition of experimental treatments. One week prior to treatments being imposed, 100 uniform sized plants were re-potted in16 litre polyethylene pots to ensure plant growth was not restricted by root volume for the duration of the trial. Treatments were imposed 16 weeks after sowing, at which point the plants were 14 weeks old from emergence and had a mean leaf number of 5.2.
Treatments
Two experiments were conducted. The first experiment involved transferring plants at regular intervals from non-inductive to inductive (vernalizing) conditions for assessment of flowering date, and the second involved comparison of flowering date between plants held under inductive conditions in a glasshouse environment and plants held under inductive conditions in a shade house. In both experiments, several possible indicators of the phase change were measured.
Temperature in the glasshouse used for non-inductive conditions varied over the duration of the experiment (Table 1). Data loggers were used to measure the air temperature and values were downloaded on to a computer using Gemini Data logger manager software. Mean maximum daytime temperature over the 8-month duration was 29.7ºC and a mean minimum temperature over the same duration was 19.8ºC. The light levels in glasshouse varied between 600 and 1500 µmol.m-2.s-1. Light intensity was measured using a line quantum sensor (LI-191SA, LI-COR®, Biosciences, USA) attached to a data logger which recorded light intensity every 30 seconds.
Inductive, vernalizing conditions were applied to plants using refrigerated growth rooms at 5 ± 1ºC. Computer controlled trolley systems transferred plants between a common glasshouse space and the three refrigerated growth rooms daily. Each of the three trolleys and associated cold chambers had the capacity to hold 15 plants. The trolleys were programmed to move into the glasshouse space at 6 am each morning and return to the adjacent refrigerated chambers at 4 pm in the afternoon. Supplemental lighting was used in the refrigerated chamber to maintain equivalent day length to ambient glasshouse conditions and was provided by combined mercury and fluorescent lights with a photon flux density of 30.2 µmol.m- 2.s-1.
Pest control measures
To control mite infestations, a predator (Phytoseiulus persimilis) was released to achieve long term control. Application of Apollo® (a.i clofentezine) and Calibre® (a. i hexythiazox) (Miticides) was required when pest population reached a point where damage to plant growth was occurring. This control measure was necessary due to problems in mite control associated with the hairy and dense nature of the foliage of P. bracteatum. To prevent white fly (Encarsia formosa) infestation, a parasitic nematode biological control agent was released on a regular basis to kill the larvae. The plants were regularly treated with Benlate (Fungaflor), a fungicide to control Fusarium which was a problem in the previous experiments conducted in the glasshouse.
Experimental design
A total of 100 plants were used for the two experiments, out of which 70 plants were kept in the glasshouse and the remaining 30 plants were kept in the shade house. For the main juvenility experiment involving plant transfers, plants were initially held in the glasshouse. Five plants were moved from the non-inductive glasshouse conditions onto trolleys to receive the chilling treatment every 3 weeks. The first transfer was carried out on April 24th, 2008, and thereafter regular transfers of 5 plants were completed at 21-day intervals. These transfers were continued until the final transfer on September 18th. At the completion of the transfers, 40 plants were held in the inductive conditions and the remaining 30 were used as control plants in non-inductive glasshouse conditions Table 2.
Measurements
Plant height, leaf number and leaf length were measured at weekly intervals after planting. Plant height was determined by measuring the distance from the base of the plant to the highest point of the rosette leaf arrangement. As stem elongation is negligible prior to flower stem development, plant height was essentially a measure of leaf length and the erectness of the leaves in the rosette structure. Leaf number was recorded for each plant but was not a measure of total number of leaves produced by each plant as some leaf loss due to leaf senescence occurred. Total leaf number was estimated from current leaf number through by addition of an estimate of leaf loss from the rate of leaf senescence. Leaf length of the youngest, fully expanded leaf was assessed using Vernier calipers and was a measure of lamina length.
A digital image of the adaxial surface of the youngest fully expanded leaf from each plant was taken each sample date using a Sony cyber-shot (Model DSC TX5P) and used to assess trichome development. As the major visible changes associated with plant ageing were in the distribution of trichomes and an increase in trichome density, a scale of measurement was developed for estimating the hairiness of leaves. Hairiness was assessed visually on a scale of 1 to 5 in order of increasing trichome density Table 3.
Two measures of flowering were recorded; the date at which the flower bud first became visible and the date that the flower bud opened and anthesis occurred. As plants were not observed daily, the precise date of flower bud appearance was not obtained. Accurate assessment of anthesis was possible as daily assessment were undertaken when each plant approached this stage.
Effect of targeted CRISPR/Cas9-facilitated deletion: To prove the efficiency of the CRISPR/Cas9-generated deletions, primers were planned upstream and downstream of various target sites, including the loci edited by the vectors pDIR21-AP1, pDIR21-SVP, and pDIR21- TFL1. The amplified fragments covered the targeted deletions in each gene. The corresponding primers are confidential. Conventionally found genome editing in these sequenced plants by PCR amplification and sequenced three clones of each PCR product cloned into a T-vector. In the AP1 lines, have obtained eight T1 lines that had the targeted deletion from 23 hygromycin-tolerant plants using a pair of AP-1/AP-3 primers. The analysis showed four types of genomic deletions at the target sites between gRNA1 and gRNA2 by DNA sequence analysis. In SVP lines, five T1 lines that had the targeted deletion were obtained from 20 hygromycin-tolerant plants, and the analysis showed three types of genomic deletions at the target sites between gRNA3 and gRNA4 by DNA sequence analysis. In TFL1 lines, 20 T1 lines that had the targeted deletion were obtained from 100 tolerant plants, and the analysis showed four types of genomic deletions at the target sites between gRNA5 and gRNA6 according to DNA sequence analysis.
Finding homozygous focused deletions in mutant lines of Papaver bracteatum: To study and confirm the homozygous mutant status of the targeted genes in the treated vernalized plants, mutations at each disrupted gene locus using three pairs of primers covering the two cut sites and the specific sites between each primer was focused. The matching primers are considered. In the PCR detection results, the absence of all three fragments was indicative of homozygous deletions in AP1, and TFL1. The middle fragment for the T2 and T4 lines of the TFL1 targeted deletion was amplified by the PCR primer pair TFL-1/TFL-3, showing that chromosomal translocations occurred between the two cut regions.
Flowering attributes of mutant Papaver bracteatum: To find the genes that regulate flowering, and changes in the shoot apical meristem during floral development, phenotypes of the untreated plants were analysed. AP1 encodes a MADS-domain transcription factor and activates floral organ identity genes to promote floral meristem formation. Targeted deletion of AP1 resulted in floral meristem development abnormally with an increased number of bracts and degenerated carpels united with sepals and petals This approach resulted in a significant increase in plant branching, usually with an additional secondary branch that maintained the indeterminate inflorescence phenotype in the double mutant lines. SVP encodes a MADS-domain transcription factor and is a flowering time regulator. However, it acts as an inhibitor of flowering at 140-day interval. Also, the absence of AP1, SVP, and TFL1 in double mutant plants caused the constant prolific production of shoot apical meristem division. Apical meristems were eventually formed and ultimately emaciated the entire branch. This phenotype is identical to that of the SVP mutant. The determinate flowering inflorescence which was formed in the double mutant was similar to that of the TFL1 mutant.
Statistical Analysis: An analysis of variance (ANOVA) was undertaken using Proc GLM in SPSS in accordance with the experimental design. Where treatments were significant, Fishers protected Least Significant Difference (LSD) was calculated to compare treatment means.
Results
Significant differences in the time of flowering were found between the transfer date treatments. Flowering was first recorded on July 24th, 27 weeks after plant emergence (Table 4). None of the plants held in non-vernalizing conditions flowered over the 56-week duration of the trial, while all the plants transferred to the vernalizing conditions had reached anthesis in the same time period. The mean period between first visible signs of the flower bud and anthesis remained constant at approximately 3 to 4 weeks irrespective of the date of transfer of plants to vernalizing conditions. This indicated that differences in flowering date between treatments could not be explained by differences in the rate of flower development after the first visible sign of the flower bud and were therefore likely to be due to differences in the timing of flower initiation.
The shortest duration between transfer to vernalizing conditions and anthesis occurred for plants transferred on June 5th and June 26th, indicating that these plants had completed the juvenile phase prior to transfer. Plants transferred on May 15th reached anthesis at a similar date to the subsequent transfer treatment, but received approximately 3 additional weeks exposure to vernalizing conditions indicating that the plants completed the juvenile phase while in the vernalizing conditions and then received the required duration of vernalization to induce flowering. The delayed flowering of plants from the first transfer date treatment compared to the two subsequent transfer date treatments may have been due to slower growth rate of the plants in the cooler, vernalizing conditions compared to the equivalent juvenile plants maintained under non-vernalizing conditions.
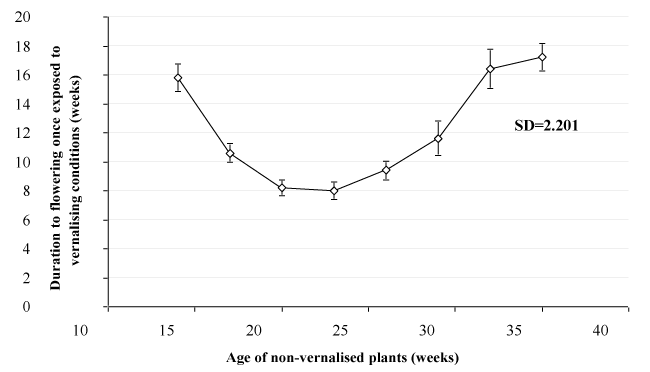
A trend of increasing age at flowering with later transfer dates to vernalizing conditions was found from the June 5th transfer treatment onwards, following a small decrease in flowering age between the first and subsequent 2 transfer dates (Figure 1). Given that an 8-week period between commencement of vernalization and flower opening was the shortest duration found, the data suggest that, under the growing conditions utilized in this experiment, juvenility was completed approximately 20 weeks after emergence.
The increase in plant age at flowering between each of the transfer dates after plants had reached maturity was greater than the 3-week interval between transfer dates. As glasshouse conditions were not constant for the duration of the trial (Table 3), it is probable that changes in conditions affected either the rate of initiation or early development of the flowers.
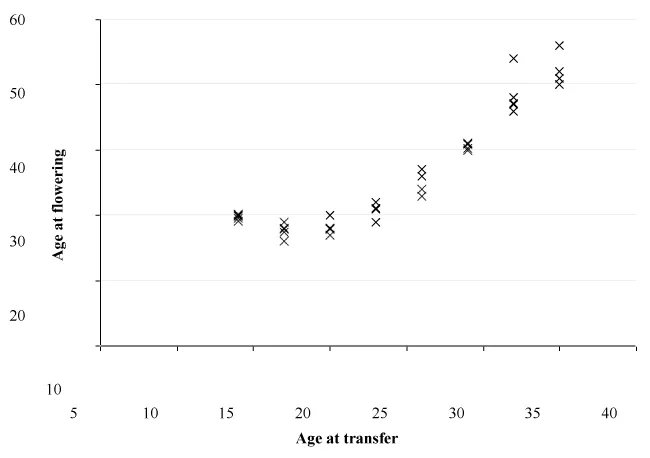
Variation in flowering date was recorded at each transfer date treatment, with replicate plants generally flowering within a 4 week window for all treatments apart from the final 2 transfer dates where one plant in each treatment displayed delayed flowering. Despite the variability in flowering response, analysis of flowering age data revealed significant differences in flowering age between treatments. The trend of increasing age at flowering with sequential transfer dates was seen for transfers after the fourth treatment (June 26th, 23 weeks), with a slope greater than 1 highlighting the delay in responding to the inductive conditions after juvenility had been completed Figure 2.
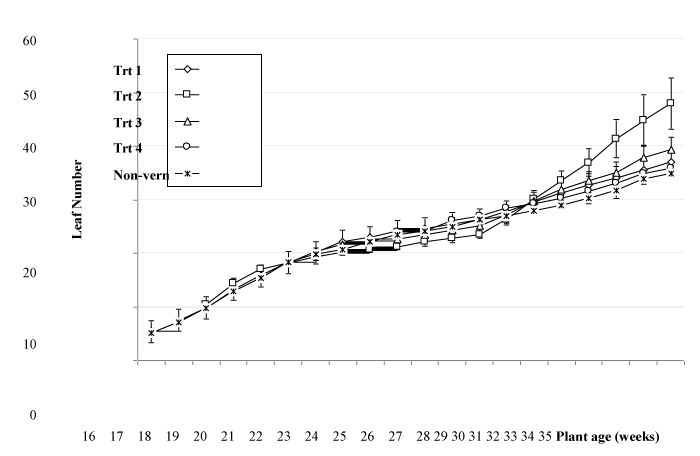
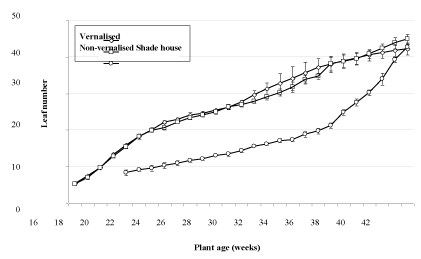
Fully expanded leaf number increased at a rate of approximately 1.6 per week between weeks 16 and 28, with a similar rate of leaf initiation found regardless of whether plants were held in inductive or non-inductive conditions (Figure 3). On the basis that the phase change from juvenile to mature plants occurred at week 20, a leaf number of approximately 16 corresponded to this change. Leaf number in 20 week old plants varied from 15.4 in non-vernalized plants, 15.8 in plants transferred to vernalizing conditions at 14 weeks old (treatment 1) and 17 in plants transferred to vernalizing conditions at 17 weeks old (treatment 2), with the differences between leaf number not being statistically significant. Leaf number increased rapidly from week 28 in treatment 2 and treatment 3 plants, with a slower increase noted for treatments 1 and 4 as well as non-vernalized plants. The increase in leaf number in treatments 2 and 3, and to a lesser extent treatment 3, occurred at the time when flower buds had been initiated and were developing on the plant.
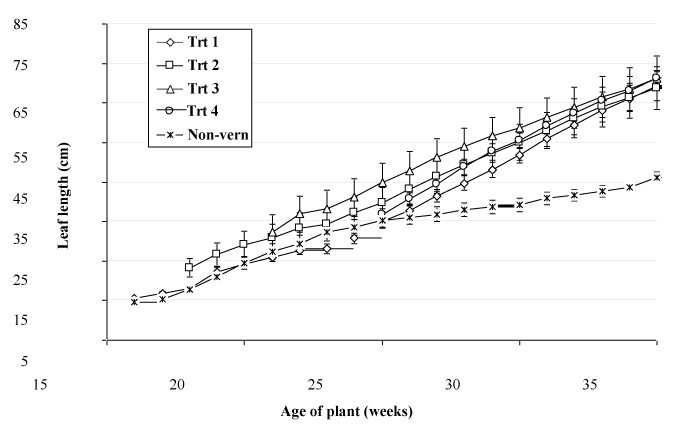
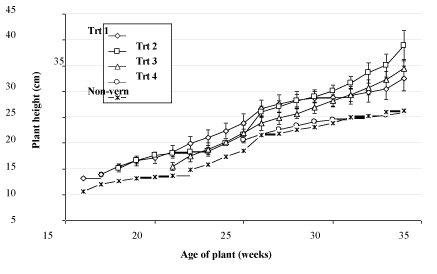
The trend in plant height was similar to that of leaf length, with an increase over time and a slower rate of increase in non-vernalized plants (Figure 4). The increase in plant height following exposure to vernalizing conditions is likely to have resulted from a combination of the increased leaf length noted above and an increased erectness of leaves observed following vernalization Figure 5.
An increase in leaf hairiness with plant age was noted during the experiment (Table 5). Plants less than 26 weeks old received a score of either 1 or 2 on the leaf hairiness scale developed during the experiment to describe trichome density and distribution on the youngest, fully expanded leaf of each plant.
A comparison between flowering times and plant morphology for plants held under the shade house conditions and both vernalising and non-vernalising conditions in the glasshouse. Flowering occurred September 21st and October 10th in plants held under shade house conditions, compared to July 16th to August 4th for plants held under vernalising conditions in the glasshouse from 14 weeks of age. Plants held in non- inductive conditions in the glasshouse did not flower Figure 6.
The period between flower buds first being visible and anthesis was between 3 and 4 weeks in shade house grown plants. As this timeframe corresponded to that noted in glasshouse grown plants, it was assumed that the duration between initiation of flowering and anthesis would be similar under both conditions. As flower initiation was found to occur 8 weeks prior to anthesis, an analysis of potential markers of the phase change in P. bracteatum at 8 weeks prior to anthesis under shade house and glasshouse conditions was undertaken.
Conditions, plants exposed to inductive conditions while still juvenile or just at the point of phase transition had between 17 and 18.3 fully expanded leaves. Plants transferred to inductive conditions when mature (transfer dates after June 5th) had a higher number of leaves at the point of initiation, reflecting the production of leaves under non-inductive conditions after the phase change had occurred. Leaf length, plant height and leaf hairiness were not useful indicators of the phase change transition as large differences were noted between plants held under shade house and glasshouse conditions.
Discussion
An extended juvenile phase of development, lasting approximately 5 months under glasshouse conditions and longer under shade house conditions, was demonstrated in P. bracteatum. The phase change from juvenile to mature growth was related to plants achieving a minimum size or stage of development, but was not linked to the age of the plant as varying growth rate by holding plants under different environments resulted in differences in the plant age at which the phase change occurred. This finding agrees with the previously published data on the juvenile phase of several woody and herbaceous species (Robinson and Wareing, 1969). While further experiments utilizing different controlled environment and field conditions, and a range of P. bracteatum germplasm, is recommended to confirm the extended duration of the juvenile phase, it was concluded on the basis of the current results that a Spring field sowing would be unlikely to lead to flowering in the first Summer season after sowing so early Autumn planting is recommended for commercial production.
The requirement for a period of vernalization was evident in the flowering responses measured in the experiments described in this chapter. Plants held under glasshouse conditions did not flower. Mean minimum temperatures in the glasshouse at the point where plants completed the juvenile phase were below 8 degrees and between 10 and 12 degrees for the following 2 months, suggesting a vernalization requirement at a lower temperature or for extended periods each night as was imposed in the inductive treatment and experienced in the shade house conditions. The increase in time taken from imposition of vernalization to anthesis with increasing plant age after the phase change was unexpected and indicated that factors other than vernalization were involved in the flower initiation and/or development processes. Exposure to high day time temperatures during vernalization has been shown to delay or prevent flowering in other species (Schwabe, 1955) and has been referred to as de-vernalization [5]. It is possible that this response also occurs in P. bracteatum, may have implication for field production of the crop in warmer climatic zones.
The end of juvenility was defined by the initiation of a critical number of leaves, with 17 mature, fully expanded leaves concluded to be a useful indicator of the phase change. Leaf number was previously reported as an indicator of phase change in some cultivars of Brassica (Sadik, 1967) while leaf number measured indirectly as number of nodes produced was identified as an indicator of phase change in tobacco [23]. Previous studies by Bradley, et al. (1997) and Adams, et al. (1998) reported that plants which develop a terminal inflorescence, the leaf number below the flower is can be used in predicting the timing of flower initiation. The CRISPR/Cas9 system has great potential for promoting functional studies in plants, as it can be easily adapted to generate loss-of-function mutations for single genes or multiple-gene clusters of unknown function and is becoming one of the most powerful tools for creating functional gene knockouts. However, a major limitation for the use of CRISPR/Cas9 in functional genomic studies in plants is the difficulty of rapidly generating and detecting stable homozygous mutations with high efficiency, as well as the inability to simultaneously mutate multiple target genes [24-35].
P. bracteatum plants contained both terminal flower stems and lateral vegetative rosette shoots at flowering, with leaf number continuing to increase during flowering through growth of the lateral shoots. Further studies under a wider range of conditions, and utilizing a range of germplasm, are recommended to confirm the applicability of leaf number as an indicator of the phase change in P. Bracteatum [35-48].
Leaf morphological characteristics used as phase change indicators in other species were shown not to be applicable in P. bracteatum. Trichome distribution and density (Kolodziejek, et al. 2006) leaf size (Kerstetter and Poethig 1998) and plant height, which were a measure of leaf erectness and leaf length, varied considerably with growing conditions and did not provide a consistent value at the phase change that could be used as an indicator.
- Martin-Trillo M, Martinez-Zapater JM (2002) Growing up fast: manipulating the generation time of trees. Current Opinion in Biotechnology 13: 151-155. Link: https://bit.ly/32MymNp
- Meilan R (1997) Floral induction in woody angiosperms. New forestry 14: 179-202. Link: https://bit.ly/2EOQcar
- Hedley CL, Harvey DM (1975) Variation in the photoperiodic control of flowering of two cultivars of Antirrhinum majus L. Annals of Botany 39: 257-263. Link: https://bit.ly/2DkvHBQ
- Hopkins W (1999) 'Introduction to plant physiology.' (Wiley: New York).
- Bernier G, Kinet JM, Sachs RM (1981) Gibberellins and gibberellin inhibitors in 'The Physiology of Flowering: Transition to Reproductive Growth' 103-114 (CRC Press: Boca Raton, FI).
- Hackett WP (1985) Juvenility, maturation and rejuvenation in woody plants. Journal of Horticultural Science 7: 109-155. Link: https://bit.ly/2QIVUNF
- Fausey BA (2005) The effects of light quantity and vernalization on growth and flowering of Arabidopsis, Achillea, Gaura, Isotoma, Lavandula and Veronica. Ph.D thesis, Michigan State University.
- Levy A, Palevitch D (1982) Effect of planting date on flowering rate and capsule yield of Papaver-bracteatum L. Experimental Agriculture 18: 305-310.
- Poethig S (1988) A non-cell-autonomous mutation regulating juvenility in maize. Nature 336: 82-83. Link: https://go.nature.com/2EXMpHw
- Sylvester AW, Parker-Clark V, Murray GA (2001) Leaf shape and anatomy as indicators of phase change in the grasses: comparison in maize, rice and bluegrass. American Journal of Botany 88: 2157-2167. Link: https://bit.ly/3bfIhPq
- Thomas B, Vince-Prue D (1997) 'Photoperiodism in plants.' (Academic Press: London, UK).
- Brewster JL (1985) The influence of seedling size and carbohydrate status of photon flux density during vernalization on inflorescence initiation in onion (Allium cepa L.). Annals of Botany 55: 403-414. Link: https://bit.ly/3lNT3RZ
- Sasamura S (1960) Studies on the relation between the plant age and the degree of sensibility to short daylength in the late paddy rice variety Zuiho. In 'Crop Science Society of Japan' 355-358. Link: https://bit.ly/32KpU1i
- Williams IH (1960) Juvenile condition and flowering in Black currant. Journal of Experimental Botany 35: 214-220.
- Borchert R (1976) The concept of juvenility in woody plants. Acta Horticulturae 56: 21-33. Link: https://bit.ly/2EVSCUu
- Greenwood MS (1995) Juvenility and maturation in conifers: current concepts. Tree Physiology 15: 433-438. Link: https://bit.ly/3hQakat
- Hackett WP (1985) Juvenility, maturation and rejuvenation in woody plants. Journal of Horticultural Science 7: 109-155. Link: https://bit.ly/32Ophn7
- Sylvester AW, Cande WZ, Freeling M (1990) Division and differentiation during normal and liguleless-1 maize leaf development. Developmental Biology 110: 985-1000. Link: https://bit.ly/34VsXX9
- Poethig RS (1990) Phase change and the regulation of shoot morphogenesis in plants. Science 250: 923-930. Link: https://bit.ly/3gPM47k
- Adams SR, Pearson S, Hadley P (2001) Improving quantitative flowering models through a better understanding of the phases of photoperiod sensitivity. Journal of Experimental Botany 52: 655-662. Link: https://bit.ly/3lEqFS0
- Katayama T (1964) Photoperiodism in the genus Oryza. Journal of Japanese Botany 18: 309-348.
- Cockshull KE (1972) Photoperiodic control of flowering in chrysanthemum. In 'Crop Process in Controlled Environments'. (Ed. AR Rees, Cockshull, K.E., Hand, D.W. and Hurd, R.G). (Academic Press: London).
- Singer SR, McDaniel CN (1986) Floral determination in the terminal and axillary buds of Nicotiana tabacum L. Developmental Biology 118: 587-592. Link: https://bit.ly/3hTWunM
- Liu Y, Gao Y, Gao Y, Zhang Q (2019) Targeted deletion of floral development genes in Arabidopsis with CRISPR/Cas9 using the RNA endoribonuclease Csy4 processing system. Link: https://go.nature.com/3gPnOC4
- Clark RJ (2002) 'Determination of seed quality for the vegetable industry.' University of Tasmania. Link: https://bit.ly/3jyUXDS
- Davidson RH, Edwards DGW, Sziklai O, El-Kassaby YA (1996) Variation in germination parameters among Pacific silver fir populations. Silvae Genetics 45: 165-171.
- Dufault RJ (1997) Determining heat unit requirements for broccoli harvest in Coastal South Carolina. Jounal of American Society of Horticultural Science 122: 169-174. Link: https://bit.ly/2YWNJSu
- Goldblatt P (1974) Biosystematic studies in papaver section oxytona. Annals of the Missouri Botanical Garden 61: 264-296. Link: https://bit.ly/34W80uY
- Gummerson RJ (1986) The effect of constant temperatures and osmotic potentials on the germination of sugar beet. Journal of Experimental Botany 37: 729-741. Link: https://bit.ly/2YU4Moc
- Khan AA (1992) Preplant physiological seed conditioning. Horticultural Review 13: 131-181. Link: https://bit.ly/3bgkfUv
- Perry KB, Sanders DC, Granberry DM, Garrett JT, Decoteau RTN, Dufault KD, Batal Mclaurin WJ (1993) Heat units, solar radiation and pepper harvest predictors. Agricultural and Forest Meteorology 65: 197-205. Link: https://bit.ly/31QWbV3
- Weibe H, Habegger R, Liebig H (1992) Quantification of vernalisation and devernalisation effects for Kohlrabi (Brassica oleracea convar. acephala var. gongylodes L.). Scientia Horticulturae 50: 11-20. Link: https://bit.ly/2EZk5o9
- Hegarty TW (1978) The physiology of seed hydration and dehydration, and the relation between water stress and the control of germination. Plant, Cell and Environment 1: 101-119. Link: https://bit.ly/3i3RRHT
- Nemeth E (1998) Selection of Poppy (Papaver somniferum L.) cultivars for culinary purposes. In 'International Medicinal and Aromatic Plants Conference on Culinary Herbs' 29-31.
- Scheinost PL, Lammer DL, Cai X, Murray TD, Jones SS (2001) Perennial wheat: the development of a sustainable cropping system for the U.S. Pacific Northwest. American Journal of Alternative Agriculture 16: 147-151. Link: https://bit.ly/2QNl6lW
- Thompson CR, Thill DC, Shafii B (1994) Germination characteristics of Sulfonylurea-resistant and susceptible Kochia (Kochia scoparia). Weed Science 42: 50-56. Link: https://bit.ly/3gRAokq
- Valdes VM, Gray D (1998) The influence of stage of fruit maturation on seed quality in tomato (Lycopersicon lycopersicum L.). Seed Science and Technology 26: 309-318. Link: https://bit.ly/2F14ZhZ
- Scheinost PL, Lammer DL, Cai X, Murray TD, Jones SS (2001) Perennial wheat: the development of a sustainable cropping system for the U.S. Pacific Northwest. American Journal of Alternative Agriculture 16: 147-151. Link: https://bit.ly/3lHCY00
- Napp-Zinn K (1987) Vernalization-environmental and genetic regulation. In 'Manipulation of flowering'. (Ed. JG Atherton) 123-132. Link: https://bit.ly/2QMylU0
- Vince-Prue D, Thomas B, Cockshull KE (1984) 'Light and the Flowering process.' (Academic Press: New York). Link: https://bit.ly/2EEsm1l
- McNicholas LF, Martin R (1984) New and experimental therapeutic role for naloxone and related opiate antagonists. Drugs 27: 81-93. Link: https://bit.ly/32HOFuU
- Davidson RH, Edwards DGW, Sziklai O, El-Kassaby YA (1996) Variation in germination parameters among Pacific silver fir populations. Silvae Genetics 45: 165-171.
- Greenwood MS, Hopper CA, Hutchison KW (1989) Maturation in larch. I. Effect of age on shoot growth, foliar characteristics, and DNA methylation. Plant Physiology 90: 406-412. Link: https://bit.ly/32Qv0Jl
- Steele MJ, Coutts MP, Yeoman MM (1989) Developmental changes in Sitka spruce as indices of physiological age I. Changes in needle morphology. New Phytology 113: 367-375. Link: https://bit.ly/31PENA7
- Martin-Trillo M, Martinez-Zapater JM (2002) Growing up fast: manipulating the generation time of trees. Current Opinion in Biotechnology 13: 151-155. Link: https://bit.ly/31MHX7F
- Halevy AH (1985) 'Handbook of flowering.' (Boca Raton: Florida, USA). Link: https://bit.ly/32Q2hnI
- Hackett WP (1985) Juvenility, maturation and rejuvenation in woody plants. Journal of Horticultural Science 7: 109-155. Link: https://bit.ly/2Ge07qs
- Bongard-Pierce DK, Evans MMS, Poethig RS (1996) Heteroblastic features of leaf anatomy in maize and their genetic regulation. International Journal of Plant Science 157: 331-340. Link: https://bit.ly/3gQzTXF
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
