International Journal of Agricultural Science and Food Technology
Antioxidant and ACE-Inhibitory Activity of Common Bean Whey Fortified Yoghurt with Assessed by in vitro Static Gastrointestinal Digestion
1College of Food Science and Technology, Nanjing Agricultural University, Jiangsu Province, P R China
2College of Animal Science and Technology, Nanjing Agricultural University, 1 Weigang Road, Jiangsu Province, P R China
Author and article information
Cite this as
Zahir A, Shen Z, Rui X, Huang J, Hamdard E, et al. (2020) Antioxidant and ACE-Inhibitory Activity of Common Bean Whey Fortified Yoghurt with Assessed by in vitro Static Gastrointestinal Digestion. Int J Agric Sc Food Technol. 2020; 6(1): 011-021. Available from: 10.17352/2455-815X.000049
Copyright License
© 2020 Zahir A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Yogurt supplemented with plant source proteins are received increased attention but few studies investigated effect of plant source proteins supplementation on protein digestibility and releasing of bioactive peptides. In this study, a residue rich in protein derived from common beans starch production, Common Bean Whey (CBW), was applied in development of novel yogurt. CBW from four major common beans varieties, namely white, black, kidney and cranberry were utilized. The novel yogurts were subjected to in vitro Gastro-Intestinal Simulation (GIS) digestion and protein digestibility, antioxidant activity and Angiotensin I Converting Enzyme (ACE) inhibitory property were studied. Compared to the control, Common Bean Whey Fortified Yogurt (CBWFY), particularly black bean whey was the predominate stable sample to protein degradation in the gastric and intestinal phases. Peptide content and SDS-PAGE revealed that CBWFY samples exhibited mostly lower hydrolysis grades in gastric and commence of duodenal phases. High antioxidant and ACE inhibitory activities results were attributed to CBWFY, in which significantly (p < 0.05) higher value was observed in kidney bean whey digesta at120min phase of intestinal digestion. For the first time, the outcomes of this investigation demonstrated the influence of four varieties of common beans whey supplementation on protein hydrolysis kinetics, digestive degree, antioxidant activity and ACE inhibitory properties on yogurt.
ABTS: 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); ACE: Angiotensin-I converting Enzyme; BCA: Bicinchoninic Acid Protein Assay; CM: Cow Milk; CBW: Common Bean Whey; CBWFY: Common Beans Whey Fortified Yogurt; DPPH: 2, 2-Diphenyl-1-Picrylhydrazyl; HA: Hippuryl Acid; HHL: Hippuryl-Histidyl-Leucine; LAB: Lactic Acid Bacteria; FRAP: Ferric Reducing Antioxidant; GIS: Gastrointestinal Simulation; TFA: Trifluoroacetic Acid; TPTZ: 2,4,6-Tripyridyl-S-Triazine; SIVD: Standardized Static in vitro Digestion; SPI: Soy Protein Isolate; SDS-PAGE: Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis; SSF: Simulated Saliva Fluid; SGF: Simulated Gastric Fluid; SIF: Simulated Intestinal Fluid; OPA: O-Phthaldialdehyde Assay; Vc Vitamin C.
Introduction
The growing role of developed and functional foods on the health benefits has experienced rapid market growth in recent years. This growth stimulated attention in the consuming of probiotic containing novel foods [1,2]. Common bean (Phaseolus vulgaris), firstly originated in Central and South America and extended to other regions of the globe, is broadly consumed in many regions [3,4].Common bean is an important group belonging to pulses along with lentil, pea and chickpea. Because of high protein content (18.5-32%) and low expense, pluses are regarded as “meat of the poor” and are a good source of plant proteins referring to huge parts of global population, especially in developing countries. Proteins of common bean are rich in lysine in comparison to cereal proteins. Furthermore, pulses contain a balanced nutritional ingredient which included considerable quantities of dietary fiber (14.6-26.3%) and low in fat (2-5%) [5-7]. Considering the composition of common bean, they contain several bioactive substances including lectins, phytates, oligosaccharides, enzyme inhibitors, and phenolic compounds that performs important metabolic roles in animals and humans [8-10]. There is growing evidence that pulses ingredients can helping in decreasing the predicted complications of severe sickness for example, cardiovascular disease, diabetes, certain forms of cancer, hypertension, obesity, osteoporosis, constipation and gastrointestinal infections, improvement of lactose metabolism, suppression of Helicobacter pylori infection, and also health beneficial effects of probiotics including antimicrobial activities [11-13].Common bean is the source of high quality starch in China. A large number of by-products (Common Bean Whey, CBW) are produced in starch production, and CBW is usually discarded. However, CBW is rich in protein, lipid, ash and also had antioxidant activity and ACE inhibitory properties.
Yogurt is a cultured dairy product with a complex gel sequence that contains protein, polysaccharides and lipids in its composition. Usually, yogurt is made by fermenting cow’s milk using a symbiotic culture Lactobacillus delbruekii subsp. bulgaricus and Streptococcus thermophillus under managed environmental conditions and temperature. The function of these starter bacteria attributed to milk acid production and the synthesis of aromatic compounds [14-17]. Lately, investigations concentrated their directions on the combination of soy protein, dairy protein, and LAB have been performed [18,19]. Various biological activities through in vivo and in vitro investigations exhibited that, the health benefits related with the consumption of yogurt and pulses ingredients are demonstrated greatly, these activities including anti-inflammatory, antigenicity, antimutagenic, anticarcinogenic and angiotensin I-converting enzyme-inhibitory activity influences [20-22]. In addition, it also exhibited that regular consumption of yogurt supplemented with pulses ingredients improves the digestive function, enhance cardiovascular health and immune system [23]. However, incorporation of pulses ingredients with proteins produce a complex resulting alterations in the function, structure and nutritional attributes and digestibility of proteins. According to the investigation by [17], the interactions between different pulses ingredients particularly Soy Protein Isolate (SPI) and dairy proteins caused an increase of lysine, leucine, isoleucine, methionine and threonine as well as enhanced essential amino acids bioaccessibility. Furthermore, the effect of fermentation pH has been studied on protein-tea polyphenols combination under GIS digestion with fermented soymilk curd [20]. Results revealed, that through in vitro digestion at pH 5.45.7, interaction of soymilk curd with tea polyphenols was effective regarding protein hydrolysis and digestibility. For clearly understanding under simulated functional environments of ingested food in human digestive system, an in vitro digestion scheme is an operative and certified method [20]. As reported by author, pepsin-treated protein hydrolysis of black bean thorough in vitro digestion demonstrated high degree of hydrolysis compared to alcalase digestion [24]. In addition, by year 2020, the world population increases in both Africa and Asia and a larger proportion is expected, there will be demanded necessities for expanding food security and protein sources, among different pulses common bean will be take a significant and considerable part [25]. Thus, it is necessary to seek pattern for supplying common bean ingredients for fortification dairy products as a component of final novel foods. Therefore, in current study, a filtered supernatant part of common beans slurry as starch extraction residue named Common Beans Whey (CBW), were used for supplementation of cow milk to produce novel Common Beans Whey Fortified Yogurt (CBWFY). However, the protein digestibility of CBWFY fermented by LAB is not well understood so far. According to our study, no investigation has been conducted to address the influence of CBW fortification and fermentation by LAB strains on the protein digestibility and bioaccessibility of the CBWFY up to date. Therefore, in the present study, CBWFY and control were subjected into gastro intestinal digestion model. The protein bioaccessibility by digestion process of CBWFY and control were operated via analyzing the peptide content, electrophoresis, evaluation antioxidant activities and ACE-inhibitory property. Outcomes of the current study will facilitate in developing the preliminary knowledge for the exploring the digestive attributes, antioxidant and ACE inhibitory activity in static stimulated digestion system of CBWFY developed novel foods.
Materials and methods
Materials
Four varieties of common bean (Phaseolus vulgaris L.), namely, white, black, cranberry and kidney were obtained from a local supermarket in Nanjing, China and stored at 4ºC before use. Lactobacillus bulgaricus was provided by China Center of Industrial Culture Collection (CICC), Beijing, China.
Preparation of common bean whey-fortified yoghurt (CBWFY)
A certified quality of common bean seeds was selected and proceeded with required procedure until ready for homogenizing, these steps were included, rinsing, then soaking with 6-folds distilled water at ambient temperature for approximately 12h, hereafter drained, dispersed and finally the slurry was produced using homogenizer BE601AB, Midea, China. 200-mesh screen cloth was used for filtration of slurry to eliminate the insoluble okara, and then to remove any precipitated starch, stored at 4ºC for about 12h. Predicated starch was removed from the system. The supernatant, Common Bean Whey (CBW), was then carefully decanted and collected. At boiling temperature, CBW was sterilized for 5min and prepared for inoculation. 75% of Cow Milk (CM) was added to CBW to make CBW -CM mixture with 25% CBW content. Fermentation was initiated by the inoculation of L. bulgaricus at ratios of 3%. Fermentations were performed at 37°C until the pH dropped to 4.60 and Common Bean Whey Fortified Yogurt (hereafter, CBWFY) was obtained.
Proximate analysis of CBW
The proximate analysis of samples (whey) were determined by using the Association of Official Analytical Chemists - AOAC (2005) methods. BCA protocol with a BCA kit (P0010, Beyotime, P. R. China) was used to determine protein content, and as standard bovine serum albumin was used. Lipid analysis was carried out following alkaline hydrolysis method referred to Association of Official Analytical Chemists.
Sensory evaluation
Sensory profiling of the samples was carried out by using descriptive sensory analysis. The sensory panel consisted of nine panelists (6 females, 3 male), selected and trained. A broad list of properties characteristic for the samples in terms of visual properties (thickness, drawing sense, brightness & smoothness), taste (refreshing degree, viscosity & delicate degree), and flavor (sour/ sweet ratio, sweet, milk taste & aftertaste) have been developed by the panel. The properties were rated with 1-9, continuous scale (0 = no perception; 9 = strong perception). Each sample was carried out in triplicate.
in vitro GIS digestion
Standardized static in vitro digestion protocol with slight changes was used to performed static in vitro gastrointestinal digestion (SIVD) of control and CBWFY [26]. Briefly, to perform buccal digestion, 3.5mL of control and CBWFY with SSF (1:1, v/v) was mixed and placed in a shaking water bath (SWB series, Biobase, Shandong, China) for 2min at 37°C with 55rpm. The subsequent gastric digestion was initiated by addition SGF at ratio 1:1 to the buccal sample and then the simulated gastric digestion was performed for 1h by shaking in water bath with 55rpm at 37°C. The pH of gastric digestion was adapted to 7.0 and at same ratio (1:1, v/v) of SIF was added to the gastric digesta. The digestion process was performed for 2h with 150 rpm at 37°C. During the digestion, eight samples were obtained from control and CBWFY, at all phases; including before digestion (P0), after oral digestion (P1), gastric digestion (P2) at 5min, 30min, and 60min and intestinal digestion (P3) at 5min, 30min and 120min were performed. To inactivate the enzyme, subsequently all samples were boiled for 5min.
Peptide content of digested samples
Peptide content of control and CBWFY at all phases of GIS digestion were determined by using OPA assay. For preparation the OPA solution, the previous method reported by [20] was used. Before measurement, obtained samples from different phases of digestion were centrifuged and filtered with at 10kDa cutoff tubes (Millipore, USA). For determination of peptide content, the filtered parts were obtained and prepared. 2mL of OPA solution and 50µL of filtered samples were taken and mixed. At room temperature, the mixed solution incubated for two min and at 340nm by using (U-4100, Hitachi Ltd., Japan) the absorbance was measured. The test was carried out in triplicate.
Electrophoresis
Protein profile of control and CBWFY samples, at all phases of GIS digestion were carried out by conducting SDS-PAGE. The experiment was performed following the previous reported protocol [20]. Stacking and separating gel with 4% and 12% concentration were used, respectively. Before electrophoresis, obtained samples from various digestion phases, were warmed in boiling temperature for 5min and in each line 20µL of sample was loaded. The electrophoresis was performed by using Bio-Rad instrument (Bio-Rad Laboratories, Inc., Hercules, CA, USA), voltage 60V was used for stacking gel and 120V for separating gel. A protein marker with molecular weight ranged (15 – 130kDa) was used, to estimate the protein subunits values. For staining the gels at room temperature, 0.1% (w/v) Coomassie brilliant blue R-250 was used. The stained gels scanned with Image Scanner III (GE Healthcare Biosciences, Uppsala, Sweden) and Quantity One software, version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for analyze.
Evaluation of antioxidant activities
DPPH assay: DPPH radical scavenging ability of control and CBWFY were measured according to the method mentioned by Xiao et al., (2015). In brief, at various concentration (0.058mg/mL), 2mL of digested samples were mixed with 2mL of DPPH reagent (0.2mM). After addition of DPPH reagent, the mixed subsequently shaken and incubated in the ambient temperature and dim place for 30min, and then the absorbance was noted at 517nm. For calculation the result following equation was used: DPPH radical scavenging capacity (%) = [1 – Asample / Acontrol] × 100. Wherein the absorbance containing test sample was calculated as a Asample, and the absorbance of blank without test sample was calculated as a Acontrol. The Vc DPPH activity was also carried out.
ABTS assay: ABTS scavenging ability of control and CBWFY digesta were performed referring to the method reported by Xiao et al., (2015). For Oxidizing and generation of ABTS. +, K2S2O8 (2.45mM) mixed with ABTS (7mM) and were stored in dark place at ambient temperature for 16 h. Prior spectrophotometer usage and attaining absorbance at 734nm of 0.70±0.02, we diluted fresh prepared ABTS·+ solution. For dilution freshly prepared ABTS solution ethanol was used and then pre-experiment was performed to verified spectrophotometer. 4mL of ABTS·+ solution was added with 1ml of test sample and blended vigorously. The mixed solution was incubated in the dim place at room temperature for 6min, and then at 734nm the absorbance was noted. Following equation was used to calculate the ABTS·+ scavenging activity: ABTS ability = [1 – Asample / Acontrol] × 100.Wherein the absorbance containing test sample was calculated as a Asample, and the absorbance of blank without test sample was calculated as a Acontrol. For comparison, the Vc ABTS scavenging ability was also measured.
FRAP assay: FRAP value of control and CBWFY digested samples were measured using the previous method described by [27] with minor amendments. By blending 100mL acetate buffer (0.3M, pH 3.6), 10mL TPTZ solution (10mM, in 40mM HCl) and 10mL ferric chloride (20mM), FRAP solution was prepared. Before use, the freshly prepared FRAP solution for the experiment was warmed at 37°C. 1mL of test sample was mixed with 5mL of freshly prepared FRAP working reagent, and the mixed solution was incubated in the dark place for 20min at 37°C. Spectrophotometer was used to recorded the absorbance of sample at 593nm against control. Various concentrations of FeSO4 H2O were used to plotted standard curve. The final values of FRAP were illustrated as µM Fe (II). Obtained higher values from FRAP assay, implying a potent antioxidant activity. Vitamin C was used as a positive control.
Hydroxyl radical scavenging ability: The hydroxyl radical scavenging capacity of control and CBWFY test samples were conducted following the previous method described by [28] with some modifications. In brief, 1mL of digested sample was blended into 1mL FeSO4 (9mM), and then 1mL H2O2 (8.8mM) was added into the mixture and blended thoroughly. Immediately, 1mL of freshly prepared salicylic acid-ethanol solution (9mM) was mixed and blended comprehensively. The mixed solution was stored for 60min at 37°C, and at 510nm the absorbance of blended sample was measured. Final values were illustrated as μg VCE/g d.w.
ACE inhibitory activity of digested samples
The ACE property of test samples were performed using previous protocol reported by [29]. In brief, for preparation of 10 μl of samples, 50μl of 2.17mM HHL, and 10μl of ACE (1.55 mU), borate buffer (100mM, including 300mM NaCl, pH 8.3) was used and mixed to commence reaction. For consecutive period the reaction was carried out for 30min at 37°C. The reaction was ended by addition of 85μl of 1 M HCl and subsequently, 20µl of sample was inserted into a ZORBAX Eclipse. Plus, C18 reversed-phase analytical column (4.60 × 250mm, 5μm particle size, Agilent). 50% (v/v) methanol in water with 0.1% Trifluoroacetic acid (TFA), was used to wash the samples for 15min at a flow rate of 0.7mL min–1. The elution was managed at 228nm. The absorbance of HA peak was calculated. Control samples were prepared without the addition of CBWFY and control digested samples, while blank samples were prepared without addition of enzyme. For calculation the ACE inhibitory activity following equation was used:
ACE inhibitory activity (%) = [(Ac – As) / (Ac – Ab)] x 100
Where Ac stand as absorbance for the control which 10 μl borate buffer was poured in substitution of sample, while Ab stand for the blank absorbance without ACE and As stand for test sample absorbance.
Statistical analysis
The statistical analysis was subjected by variance (ANOVA) using Duncan’s multiple comparison tests and differences were considered to be significant at (p < 0.05) level using IBM SPSS Statistic software.
Results and discussion
Proximate composition of CBW
As shown in Table 1, regarding protein content, no significant (p < 0.05) difference was recorded between CM and CBW, whereas significantly (p < 0.05) lower value of lipid contents were obatined for samples CBW. This indicated that the CBW is appropriate supplementary ingerdeints for development of novel food. Significantly (p < 0.05) higher value of moisture and ash contents were attributed to CBW than CM, except lower value of ash content was obtained with white bean whey.
Peptide content and electrophoresis under GIS digestion
Figure 1 shows the result of peptide content of the samples collected before and during the GIS digestion. Peptide contents were linked with digestion and increased for all test samples as the GIS digestion prolonged. No significantly (p < 0.05) increase was observed before digestion (P0), P1 and P2 (P2-5, P2-30) phases of GIS digestion. Slightly increment was observed for all samples during 60min of the gastric phase (P2-60), which might have attributed to some extent breakdown by enzymatic activity of pepsin. Our results findings were similar with result obtained by previous authors [30]. The peptide content of digested CBWFY increased at the beginning phase of duodenal digestion(P3-5), while until P3-120, a considerable increment from control was not observed. This might be moderately due to the existence of digestive enzymes in duodenal digestion. At the end of duodenal digestion, CBWFY sample exhibited the highest value of peptides, particularly for CM fortified with kidney bean whey (2.03mg/mL), followed by black (1.70mg/mL) and then cranberry (1.50mg/mL) respectively. The results indicated that CM fortified with four varieties of common bean whey influence the generation of peptides from CBWFY matrix. Remarkably, the CM fortified with kidney bean whey presented the maximum peptide content compared to the other three samples. Results proposed that a higher proportion of short peptides were produced by CBWFY than control accredited to the hypothesis that strong force gravitation could be happened between cow milk and CBW proteins during fermentation and 24h cold storage, and finally affecting the hydrolysis during GIS digestion.
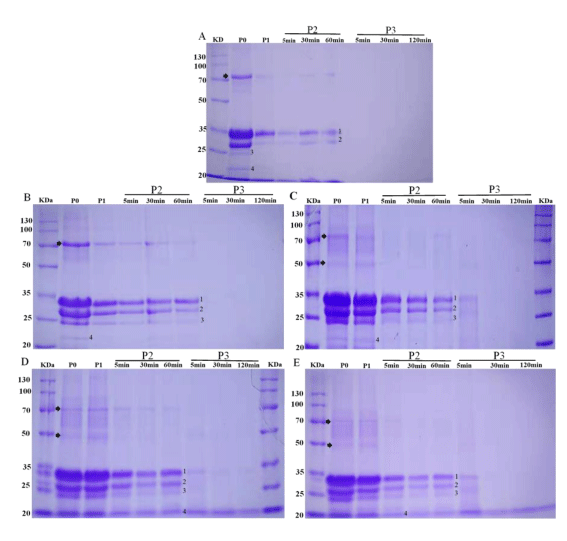
The SDS-PAGE was used to detect the hydrolysis profile of CBWFY and control shown in Figure 2. The main proteins of Phaseolus L. beans are salt-soluble globulins, including a major portion of vicilin and a slight fraction of legumin [31]. Vicilin is a 7S globulin and is frequently attributed as phaseolin. It represents 50% of the total protein content and contained 3–5 subunits. While, legumin included of acid and basic subunits and has 11–12S globulin, usually sediments with vicilin as a single fragment [32]. However, casein and whey are the major proteins of cow milk. Casein possess four different subunits (αs1-CN, αs2-CN, β-CN, κ-CN), while whey proteins comprise the β-lactoglobulin (β-LG), α-lactalbumin (α-LA) and milk serum albumin [21].
All samples showed a number of polypeptide subunits were observed in control and CM fortified with CBW including casein (α, β and κ subunits) and 7S vicilin subunits of molecular weight (MM) between 76 and 17 kDa with main subunits of 49, 48, 47, 33, 29, 28 and 20 kDa. Compared to the control, in CBWFY samples significant changes were observed when CBW supplementation were used (Figure 2B-E), indicating that, cross linking of proteins was occurred, thus more bands were appeared. According to the studies [33], in cow milk protein the αS1- and β-CNs, the bound possess phosphate groups effects several functional attributes to the cow milk proteins, such as their digestion process, bioaccessibility and immunogenicity. The author also mentioned that, CNs containing divalent bound phosphate groups were corresponding for the reduction digestibility of this protein, which is supporting our observation, since 75% of CBWFY is cow milk. These four yogurts showed more intensives bands at P0 (Figure 2B-E), implying that the main portions were not degraded in the gels. After the buccal and gastric digestion P1 and P2 (P2-5, P2-30, P2-60), the predominant bands could be observed in all CBWFY samples (Figure 2B-E), which were predicted to be aggregation between cow milk protein (casein) and 7S vicilin. This might be probably due to the aggregations of proteins make bands resistance to enzymatic hydrolyse. However, the intensity of bands corresponding to α, β and κ casein of all sample decreased throughout the oral and gastric digestion, but in three samples were still visible at initial phase of duodenal digestion (P3-5, Figure 2C-E). This diminution in the band intensity could be due to the dilution of stimulated to the saliva and breakdown by enzymes and stimulated gastric juice particularly for control sample, which the reduction was more than CBWFY samples [34]. This can be attributed that the enzymatic activity, that breakdown control protein easily during GIS digestion. These results were similar from those who obtained by [24], in which up to 60min hydrolysis bands corresponding to the vicilin and legumin are visible, suggesting some accumulation of proteins happened by hydrogen bonds, thus in CBWFY samples more intensive bands were observed. In addition, the intensity of bands was more for sample black followed by kidney, cranberry and white common bean whey fortified yogurts (Figure 2B-E). The bands confrontation or exposure to digestion depends on the structural features of each protein. For instance, presence a high quantity of β-sheet organizations, usually for 11S and 7S subunits had difficulty to accessing proteolytic enzymes [35]. Regarding to the four yogurts, the band at molecular weight of approximately 72 kDa exhibited reduction in band concentration and vanished toward the end of gastric digestion (Figure 2A-D). Moreover, at P0 and P1 the bands corresponding to 7S vicilin with molecular weight 47 to 49 kDa in yogurt fortified with black, kidney and cranberry bean whey were visible, except for sample white bean whey.
The subsequent duodenal digestion caused rapid protein breakdown for major protein of the control (casein and β- lactoglobulin) and common beans (7S vicilin and 11s legumin subunits) disappeared within 5min of intestinal digestion and no integral bands could be gained throughout this stage. In particular, the intensity of the β-CN and κ-CN and subunits of beans dropped rapidly at 5min of duodenal stage. This suggested that those proteins in CBWFY and control yogurts were degraded into small peptides, showing that the enzymatic reactivity was much higher. However, in the intestinal stage, samples showed extra bands which wasn’t visible in control and white bean whey (Figure 2C-E, P-3) at MM of 32, 31, 26, and 17 kDa, that may similar to the α, β, κ casein and basic subunits, accordingly.
Antioxidant capacity of CBWFY under GIS digestion
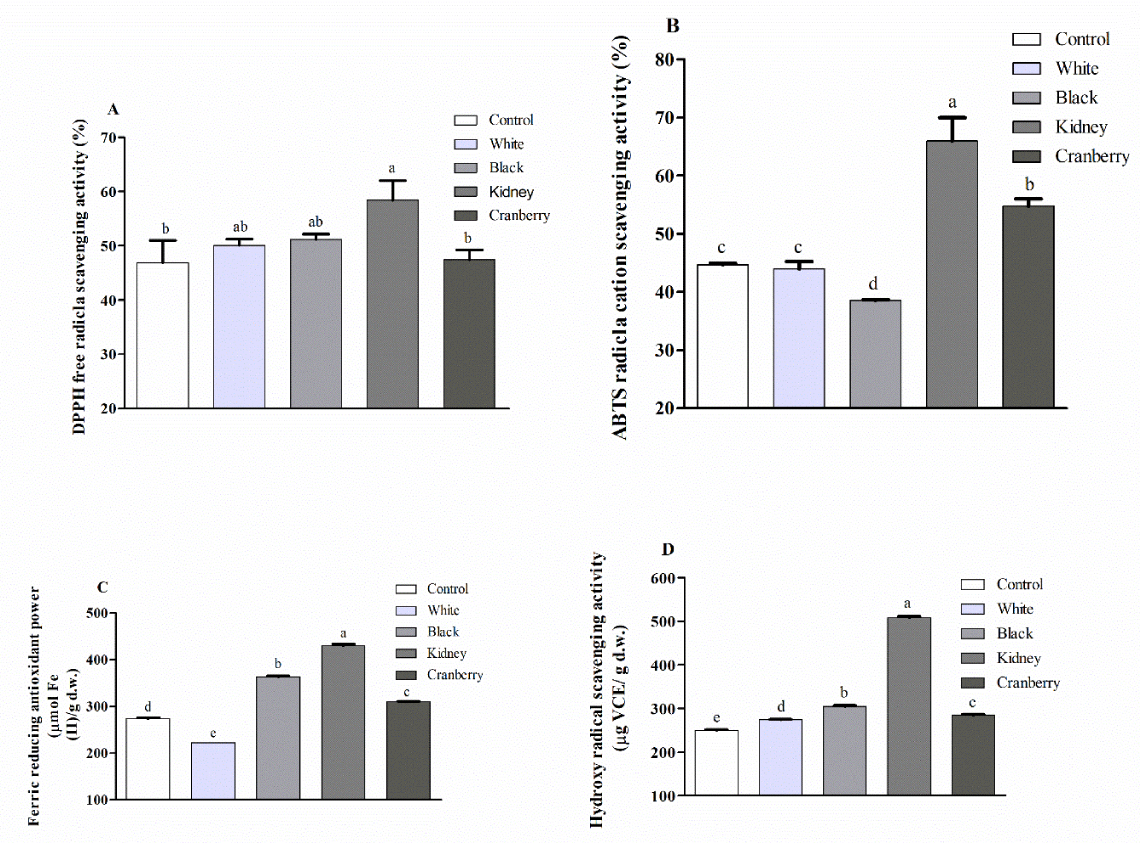
Many factors influenced the antioxidant activity, and for measuring antioxidant activity a number of usual methods are on the ground that have their privilege and drawback, consequently specific method cannot adequately explain the antioxidant capability of food complex [28]. Therefore, many antioxidant capacity analyses with several methods and mechanisms were performed to replicate the antioxidant capacities of control and CBWFY, as shown in Figure 3A-D.
DPPH assay: Antioxidant activity of CBWFY and control at P3-120 phase of duodenal digestion measured by DPPH. was shown in Figure 3A. Based on the result, the values obtained from CBWFY samples after duodenal digestion revealed (p < 0.05) higher DPPH. activity compared to the control sample, suggesting that CBWFY are more efficiently improved the antioxidant activity than control during duodenal digestion. Among four CBWFY, kidney bean whey showed significantly (p < 0.05) higher value of DPPH. activity followed by black, white and cranberry samples. Compared to the control, maximum antioxidant activity of CBWFY can be attributed to high amount of hydrophobic groups. Prior to the protein hydrolysis, the hydrophobic groups were considered to be concealed into the tertiary and quaternary protein structure. In addition, it might be contributed that enzyme breakdown the protein’s containing high molecular weight. According to the investigations by [24], after two hours pepsin-treated protein hydrolysis of Azufrado bean (Phaseolus vulgaris L.), showed 44% inhibition of DPPH. which being inferior than the result noted for the current investigation. The result also exhibited that GIS digestion breakdown high molecular weight proteins into peptides which act as free radical scavenging resulted enhanced the antioxidant activity of CBWFY.
ABTS assay: The ABTS.+ scavenging ability result of control and CBWFY at P3-120 phase of duodenal digestion were shown in Figure 3B Based on ABTS.+ scavenge radical capacity result, again CBWFY showed higher scavenging ability as compare to control, regardless for sample black bean whey which low value was obtained, suggesting that CBWFY had better antioxidant ABTS.+ radical scavenging ability comparing to control. In terms of four CBWFY, kidney bean whey demonstrated significantly (p < 0.05) higher scavenging activity followed by cranberry, white and black samples. The peak antioxidant activity displaying by the CBWFY after P3-120 phase of duodenal digestion, addressed by the this assay is accredited to the connecting capability of hydrophobic proteins with ABTS.+ hydrophilic radicals [24]. This result is similar with finding [24], that up to two hours black bean protein hydrolysates showed 73% inhibition of ABTS.+ scavenging activity. Obtained result, suggests that enzymatic activity during GIS digestion capable to generate high amount of antioxidant fractions from CBWFY, probably due to its higher proteolytic activity on this food matrix.
Assay of FRAP: In light of the result, after P3-120 phase of duodenal digestion the FRAP values of CBWFY compared to the control were higher, regardless for CM fortified with white bean whey (Figure 3C). In terms of the CBWFY, CM fortified with kidney bean whey exhibited significantly (p < 0.05) higher value of FRAP followed by black, cranberry and white samples. This suggested that the supplementation of CM with CBW enhanced the FRAP of CBWFY during fermentation and duodenal digestion. Previous studies by [36], demonstrated that fermented legume products with Bacillus sp. showed higher FRAP activity which might have direct link with the production of iron chelated components during fermentation period. The author also declared that the antioxidant capacity (FRAP) might probably be due to the presences of phenolics content in the test samples caused the reduction from TPTZ–Fe3+ compound into TPTZ–Fe2+ structure. Thus, superior phenolic content of CBWFY might ascribed to higher FRAP of CBW. Our finding is similar with previous investigation, since the phenolic content of samples was not determined in the current study.
Hydroxyl radical scavenging ability: As shown in Figure 3D, CBWFY samples demonstrated strong hydroxyl radicals scavenging activity compared to the control sample. In terms of four CBWFY, CM fortified with kidney bean whey revealed significantly (p < 0.05) higher hydroxyl radical scavenging capability. Higher values of hydroxyl radicals scavenging attributed to the CBWFY particularly for sample kidney bean might be due to their greater phenolics contents. It seems that kidney bean whey might contain more hydroxyl radical scavenging ingredients which are created during fermentation. Plenty investigations have revealed that phenolics and isoflavones demonstrate hydroxyl radical scavenging activity [36]. Earlier insights have reported that the presence of phenolic compounds plays an imperative role in sample as a ferrous ion chelating agents [37].
To conclude, CBWFY exhibited higher antioxidant activity compared to the control through different methods and mechanisms of antioxidant activity measurement. However, in terms of four CBWFY, CM fortified with kidney bean whey demonstrated higher antioxidant activity which was significantly (p < 0.05) higher than three other and control. Higher antioxidant activity in CBWFY might be attributed to their phenolic content of CBW. In addition, [38] reported that colored beans have greater antioxidant and antiradical attributes than less colored beans. Thus, this finding further support our observation which CM fortified with white bean whey exhibited low value of antioxidant activity as compare to reaming three varieties that measured via different methods.[39] also reported that kidney bean extract showed higher antioxidant activity than those from white bean extract .
ACE inhibitory properties
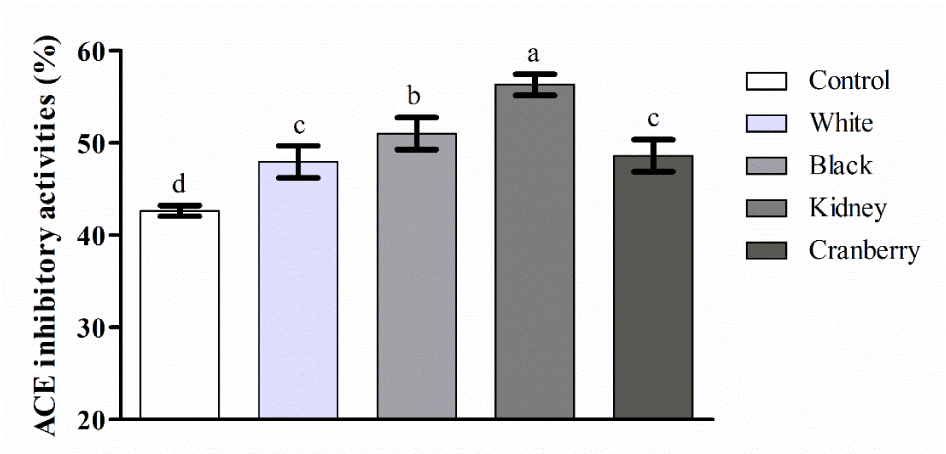
For prediction the destiny of the ingested ACE inhibitory peptides, in vitro GIS digestion is an advantageous scheme [40]. ACE inhibitory activity of CBWFY and control after P3-120 phase of duodenal digestion was shown in Figure 4. Based on the result, all samples of CBWFY showed (p < 0.05) superior ACE inhibitory activity comparing to control, indicating that CBW supplementation enhanced the ACE inhibitory activity of final product. This is might due to hydrolyse of cow milk and common bean whey proteins, mainly casein and 7S vicilin into peptides during fermentation and further hydrolyzed by GIS digestion into smaller peptides. Among all four CBWFY, the ACE inhibitory activity of kidney bean whey was higher followed by black, cranberry and white samples. This result is in accordance with the peptides content in current study. It seems that the most bioactive peptides were produced at duodenal digestion, especially for samples CBWFY. Therefore, CM fortified with CBW increased the production of bioactive peptides [41]. Reported that in fermented dairy product, generation of special proline endopeptidase enzymes such as (PepP, PepR, PepX) during fermentation, will enhances the amount of proline containing C-terminal peptides which directly linked with the ACE-inhibitory activity. A study investigated the effect of enzymatic hydrolysis on goat and cow milk and found that as the hydrolysis extended, the enzyme activity continuously breakdown the peptides, and consequently a number of amino acids at the C- or N-terminal produced that usually have strong affiliation with the active site of ACE [42]. These results demonstrated that after the treatment of CBWFY and control samples with the gastrointestinal enzymes, novel ACE inhibitory peptides were produced, which proposed that this product might have health promoting effect when reached to the digestive system.
Sensory evaluation of CBWFY
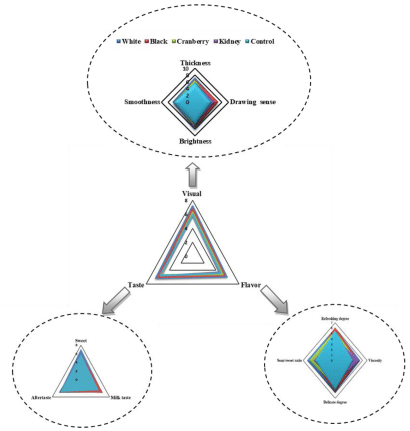
The results of sensory evaluation are shown in Figure 5. The visual indicative parameters including thickness, drawing sense, brightness and smoothness which representing the visual properties of products. The visual score for CM supplemented with CBW was higher, except similar score was obtained for CM supplemented with kidney bean whey due to low score for smoothness and similar score for thickness in comparison to CM. Among different varieties of CBW, the visual score was higher for CM supplemented with white bean whey followed by black, cranberry and kidney. Our result showed that the CM supplemented with CBW had better visual due to a good gel system resulting in a high score for thickness, drawing sense and brightness. This indicated that the CM supplemented with white bean whey was more acceptable due to high score for thickness, brightness and smoothness which also probably related to white skin, while the visual result for CM supplemented with kidney bean whey was less acceptable resulted in a low score for thickness and smoothness probably due to skin color. Previous studies also reported that, yogurt supplementation by soy protein isolate the visual properties was looks like mayonnaise [43].
The values related to taste including sweet, milk taste and aftertaste that represents the taste of novel product. Compared to CM, the taste score for CM supplemented with CBW was higher, except low score was obtained for CM supplemented with cranberry bean whey due to low score for milk taste and aftertaste parameters. This could be probably due to incomplete fermentation. Our result demonstrated that CM supplemented with CBW stimulate fermentation process as well. Among all varieties of CBW, the taste score was higher for CM supplemented with white bean whey followed by black, kidney and cranberry. Our result exhibited that the CM supplemented with white bean whey was more acceptable due to high score for sweet and aftertaste, whereas the taste result for CM supplemented with cranberry bean whey had less acceptability which resulted in a low score for sweet and aftertaste and might be due to increased acidity. According to [43,44], they have reported that, fermented milks fortified to 18% total solids showed higher taste than control sample. Taste problems in common bean whey yogurts attributed to fermented dairy aroma and common bean whey flavor which was also due to sharp lactic acid production. Another study showed that, reduction in lactose concentrations is associated with decreases in sensory sweetness insights.
Finally, all indicative parameters related to flavor including refreshing degree, viscosity, delicate degree and sour/sweet ratio which indicate flavor of final product. The flavor score was higher for CM supplemented with CBW in comparison to CM, except low score was obtained for CM supplemented with kidney bean whey, due to low score for refreshing degree and delicate degree. This implies that supplementation with CBW improved the product flavor. Regarding all varieties of CBW, the flavor score was higher for CM supplemented with white bean whey followed by black, cranberry and kidney. This result showed that CM supplemented with white bean whey was more acceptable due to high score for viscosity, delicate degree and sour/sweet ratio which also related to the taste result, while the result for CM supplemented with kidney was less acceptable regarding low score for refreshing degree, viscosity, delicate degree and sour/sweet ratio.
Among different varieties of CBW, the three indexes for sensory evaluation taste such as visual properties, taste and flavor were significantly (p < 0.05) higher for CM milk supplemented with white bean in comparison to remaining three varieties. This result is in accordance with syneresis of white bean at 5h fermentation, 25% CBW and 3% inoculation ratio that showed low whey separation which also related to response surface methodology (RSM) result represented lowest syneresis (0.27%) at 25% CBW supplementation. Further advanced investigations are suggested to explore the protein bioaccessibility, antioxidant and ACE inhibitory activity of common beans whey fortified yogurt (CBWFY) to evaluate the nutritional, antioxidant activity and ACE property value of this product.
Conclusion
In the current investigation, we studied the protein digestibility, antioxidant activity and ACE inhibitory properties of yogurt fortified with four varieties of CBW (namely white, black, kidney and cranberry) and control were assessed by using an “standardized static in vitro gastrointestinal digestion” procedure. Results showed that, compared to the control, common beans whey fortified yogurt (CBWFY) had lower protein digestibility at gastric phase particularly for CM fortified with black bean which was confirmed with peptide and electrophoresis results. The lower digestibility indicated that protein aggregation between CM and CBW proteins were occurred. All samples of CBWFY exhibited higher antioxidant activity, particularly for CM fortified with kidney bean whey demonstrated higher antioxidant activity which was significantly (p < 0.05) higher than three other and control. However, CBWFY, showed high ACE inhibitory activities and higher (p < 0.05) value was obtained for CM fortified with kidney bean whey, which is in accordance with the peptide content results. In summary, our study could be a good contribution for better utilization of CBW as efficient and valuable components for development of novel foods. Therefore, in vitro analysis of anti-nutritional factors of CBWFY could be addressed hereafter.
This work was financially supported by the Fundamental Research Funds for the Central Universities (KYZ201745). We are also grateful to the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Provincial Double-Creative Doctorate Plan.
- Mousavi M, Heshmati A, Garmakhany AD, Vahidinia A, Taheri M, (2019) Optimization of the viability of Lactobacillus acidophilus and physico-chemical, textural and sensorial characteristics of flaxseed-enriched stirred probiotic yogurt by using response surface methodology. LWT 102: 80-88. Link: http://bit.ly/3ayqyRR
- Granato D, Branco GF, Nazzaro F, Cruz AG, Faria JAF (2010) Functional foods and nondairy probiotic food development: trends, concepts, and products. Compr Rev Food Sci Food Saf 9: 292-302. Link: http://bit.ly/38hSZll
- Moyib OK, Alashiri GO, Adejoye OD (2015) Chemometric dissimilarity in nutritive value of popularly consumed Nigerian brown and white common beans. Food Chem 166: 576-584. Link: http://bit.ly/2Ti7y3X
- Granito M, Frias J, Doblado R, Guerra M, Champ M, et al. (2002) Nutritional improvement of beans (Phaseolus vulgaris) by natural fermentation. Eur Food Res Technol 214: 226-231. Link: http://bit.ly/2Tg3t0k
- Al-Numair KS, Ahmed ESB, Al-Assaf AH, Alamri MS (2009) Hydrochloric acid extractable minerals and phytate and polyphenols contents of sprouted faba and white bean cultivars. Food Chemistry 113: 997-1002. Link: http://bit.ly/38iluPC
- Vargas-Torres A, Díaz PO, Tovar J, Paredes‐López O, Ruales J, et al. (2004) Chemical Composition, Starch Bioavailability and Indigestible fraction of Common Beans (Phaseolus Vulgaris L.). Starch - Stärke 56: 74-78. Link: http://bit.ly/3ctrCrF
- Wu H, Rui X, Li W, Chen X, Jiang M, et al. (2015) Mung bean (Vigna radiata) as probiotic food through fermentation with Lactobacillus plantarum B1-6. LWT-Food Science and Technology 63: 445-451. Link: http://bit.ly/2VAr0dY
- Campos-Vega R, Reynoso-Camacho R, Pedraza-Aboytes G, Acosta-Gallegos JA, Guzman-Maldonado SH , et al. (2009) Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.). J Food Sci 74: T59-T65. Link: http://bit.ly/3ar3cNB
- Granito M , Alvarez G (2006) Lactic acid fermentation of black beans (Phaseolus vulgaris): microbiological and chemical characterization. J Sci Food Agric 86: 1164-1171. Link: http://bit.ly/32IlwiG
- Marquezi M, Maria Gervin V, Bertoldi Watanabe L, Moresco R, Regina Amante E (2017) Chemical and functional properties of different common Brazilian bean (Phaseolus vulgaris L.) cultivars. Braz J Food Technol 20 Link: http://bit.ly/39kbBCn
- López-Barrios L, Antunes-Ricardo M, Gutiérrez-Uribe JA (2016) Changes in antioxidant and antiinflammatory activity of black bean (Phaseolus vulgaris L.) protein isolates due to germination and enzymatic digestion. Food Chem 203: 417-424. Link: http://bit.ly/2vp1Nsn
- Wang N , Toews R (2011) Certain physicochemical and functional properties of fibre fractions from pulses. Food Res Int 44: 2515-2523. Link: http://bit.ly/2IaWJdR
- Zare F, Boye JI, Champagne CP, Orsat V, Simpson BK (2013) Probiotic milk supplementation with pea flour: Microbial and physical properties. Food and Bioprocess Technology 6: 1321-1331. Link: http://bit.ly/2TC4pLg
- Amakoromo E R, Innocent-Adiele HC, Njoku HO (2012) Microbiological quality of a yoghurt-like product from african yam bean. J Natural Sci 10: 6-9. Link: http://bit.ly/3cvVVhJ
- Rui X , Wang M, Zhang Y, Chen X, Li L, et al. (2017) Optimization of soy solid‐state fermentation with selected lactic acid bacteria and the effect on the anti‐nutritional components. J Food Process Preserv 41: e13290. Link: http://bit.ly/3csfqrm
- Seçkin AK , Baladura E (2012) Effect of using some dietary fibers on color, texture and sensory properties of strained yogurt. GIDA 37: 63-69. Link: http://bit.ly/2wmtkdV
- Akin Z , Ozcan T (2017) Functional properties of fermented milk produced with plant proteins. LWT 86: 25-30. Link: http://bit.ly/2vASi9j
- Nguyen TT , Bhandari B, Cichero J, Prakash S (2015) Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res Int 76: 348-358. Link: http://bit.ly/3cpDFpT
- Rui X, Zhang Q, Huang J, Li W, Chen X , et al. (2019) Does lactic fermentation influence soy yogurt protein digestibility: a comparative study between soymilk and soy yogurt at different pH. J Sci Food Agric 99: 861-867. Link: http://bit.ly/2Tg4oOc
- Xing G, Rui X, Wang D, Liu M, Chen X, et al. ( 2017) Effect of fermentation pH on protein bioaccessibility of soymilk curd with added tea polyphenols as assessed by in vitro gastrointestinal digestion. J Agric Food Chem 65: 1125-11132. Link: http://bit.ly/38d0slC
- Xing G, Lucia Giosafatto CV, Rui X, Dong M, Mariniello L, et al. (2019) Microbial transglutaminase-mediated polymerization in the presence of lactic acid bacteria affects antigenicity of soy protein component present in bio-tofu. Journal functional foods 53: 292-298. Link: http://bit.ly/38ipVKg
- Li SN, Tang SH, He Q, Hu JX, Zheng J (2020) In vitro antioxidant and angiotensin-converting enzyme inhibitory activity of fermented milk with different culture combinations. J Dairy Sci 103: 1120-1130. Link: http://bit.ly/2IawtjE
- Codină GG, Franciuc SG, Mironeasa S (2016) Rheological characteristics and microstructure of milk yogurt as influenced by quinoa flour addition. Journal of food quality 39: 559-566. Link: http://bit.ly/32QuL0n
- Evangelho JAD, Vanier NL, Pinto VZ, Berrios JJ, Dias ARG, et al. (2017) Black bean (Phaseolus vulgaris L.) protein hydrolysates: Physicochemical and functional properties. Food Chem 214: 460-467. Link: http://bit.ly/2uRq1uY
- Moyib OK, Alashiri GO, Adejoye OD (2015) Chemometric dissimilarity in nutritive value of popularly consumed Nigerian brown and white common beans. Food chem 166: 576-584. Link: http://bit.ly/2Ti7y3X
- Qing S, Zhang Q, Li W, Azarpazhooh E, Simpson BK , et al. (2019) Effects of different satiety levels on the fate of soymilk protein in gastrointestinal digestion and antigenicity assessed by an in vitro dynamic gastrointestinal model. Food Funct 10: 7855-7864. Link: http://bit.ly/2VEF6Lb
- Xiao Y, Rui X, Xing G, Wu H, Li W, et al. (2015) Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L). J Funct Foods 16: 58-73. Link: http://bit.ly/39mhkHS
- Xiao Y, Fan J, Chen Y, Rui X, Zhang Q, et al. (2016) Enhanced total phenolic and isoflavone aglycone content, antioxidant activity and DNA damage protection of soybeans processed by solid state fermentation with Rhizopus oligosporus RT-3. RSC Advances 6: 29741-29756. Link: https://rsc.li/2Ik99Qp
- Rui X, Wen D, Li W, Chen X, Jiang M, et al. (2015) Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct 6: 622-629. Link: http://bit.ly/2PGTizs
- Qing S, Zhang Q, Li W, Azarpazhooh E, Simpson BK , et al. (2019) Effects of different satiety levels on the fate of soymilk protein in gastrointestinal digestion and antigenicity assessed by an in vitro dynamic gastrointestinal model. Food Funct 10: 7855-7864. Link: http://bit.ly/2VEF6Lb
- Rui X, Boye JI, Ribereau S, Simpson KB, Prasher SO (2011) Comparative study of the composition and thermal properties of protein isolates prepared from nine Phaseolus vulgaris legume varieties. Food Res Int 44: 2497-2504. Link: http://bit.ly/2x31Lql
- Wani IA, Sogi DS, Shivhare US, Gill BS (2015) Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates. Food Research International 76: 11-18. Link: http://bit.ly/2PFtwvA w
- Salami M, Yousefi R, Ehsani MR, Dalgalarrondo M, Chobert JM, et al. (2008) Kinetic characterization of hydrolysis of camel and bovine milk proteins by pancreatic enzymes. International Dairy Journal 18: 1097-1102. Link: http://bit.ly/2wmPLzO
- Qing S, Zhang Q, Li W, Azarpazhooh E, Simpson BK , et al. (2019) Effects of different satiety levels on the fate of soymilk protein in gastrointestinal digestion and antigenicity assessed by an in vitro dynamic gastrointestinal model. Food Funct 10: 7855-7864. Link: http://bit.ly/2VEF6Lb
- Montoya CA, Lalles JP, Beebe SE (2010) Phaseolin diversity as a possible strategy to improve the nutritional value of common beans (Phaseolus vulgaris). Food Research International 43: 443-449. Link: http://bit.ly/2wmD73F
- Xiao Y, Wang L, Rui X, Li W, Chen X, et al. (2015) Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. Journal of Functional Foods 12: 33-44. Link: http://bit.ly/2TvcEcd
- Shahidi F , Chandrasekara A (2013) Millet grain phenolics and their role in disease risk reduction and health promotion: A review. Journal of Functional Foods 5: 570-581. Link: http://bit.ly/3cjGrgq
- Akond A SM, Khandaker L, Berthold J, Gates L, Peters K, et al. (2011) Anthocyanin, total polyphenols and antioxidant activity of common bean. Am J Food Technol 6: 385-394. Link: http://bit.ly/38dKeIR
- García-Lafuente A, Moro C, Manchón N, Gonzalo-Ruiz A, Villares A, et al. (2014) In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chem 161: 216-223. Link: http://bit.ly/3aoCiWY
- Rui X, Wen D, Li W, Chen X, Jiang M, et al. (2015) Enrichment of ACE inhibitory peptides in navy bean (Phaseolus vulgaris) using lactic acid bacteria. Food Funct 6: 622-629. Link: http://bit.ly/2Tw65pL
- Gonzalez-Gonzalez CR, Tuohy KM, Jauregi P (2011) Production of angiotensin-I-converting enzyme (ACE) inhibitory activity in milk fermented with probiotic strains: Effects of calcium, pH and peptides on the ACE-inhibitory activity. Int Dairy J 21: 615-622. Link: http://bit.ly/2PXkg6r
- Shu G, Huang J, Bao C, Meng J, Chen H, et al. (2018) Effect of Different Proteases on the Degree of Hydrolysis and Angiotensin I-Converting Enzyme-Inhibitory Activity in Goat and Cow Milk. Biomolecules 8. Link: http://bit.ly/38o4GHn
- Drake MA , Chen XQ, Tamarapu S, Leenanon B (2000) Soy protein fortification affects sensory, chemical, and microbiological properties of dairy yogurts. J Food Sci 65: 1244-1247. Link: http://bit.ly/2IkpWTr
- Schmidt RH, Sistrunk CP, Richter RL, Cornellet JA (1980) Heat treatment and storage effects on texture characteristics of milk and yogurt systems fortified with oilseed proteins. J Food Sci 45: 471-475. Link: http://bit.ly/32Mx0BG
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
