International Journal of Agricultural Science and Food Technology
Leveraging Environmental DNA Meta barcoding to Explore Agro-biodiversity, Microbial Dynamics and the Impact of Plant Extracts on Pest Management in Agricultural Ecosystems
Abdullah Al Mamun*, Shabiha Tasbir Rahman and M Mizanur Rahman
Department of Biotechnology and Genetic Engineering, Faculty of Biological Science, Islamic University, Kushtia-7003, Bangladesh
Cite this as
Mamun AA, Rahman ST, Rahman MM. Leveraging Environmental DNA Meta barcoding to Explore Agro-biodiversity, Microbial Dynamics and the Impact of Plant Extracts on Pest Management in Agricultural Ecosystems. Int J Agric Sc Food Technol. 2025;11(2):038-044. Available from: 10.17352/2455-815X.000227Copyright License
© 2025 Mamun AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Sustainable pest management is vital for promoting agricultural productivity while preserving environmental health. This study investigated the integration of plant-based pest control methods with environmental DNA (eDNA) metabarcoding to develop eco-friendly pest management strategy. Soil, plant, and air samples were collected from organic, agroecological, and conventional farms across Bangladesh. These samples were used to analyze the microbial and pest diversity. Plant extracts from neem (Azadirachta indica), garlic (Allium sativum), and tobacco (Nicotiana tabacum) were prepared and tested against Helicoverpa armigera under greenhouse conditions at concentrations of 10%, 25%, and 50%. eDNA metabarcoding results revealed that organic farms exhibited the highest microbial diversity (Shannon index = 3.87), while conventional farms recorded the highest pest species diversity (species richness = 27). Pest mortality rates varied by concentration, with neem extract at 50% concentration achieving the highest mortality rate (91.3%), followed by garlic (85.7%) and tobacco (78.5%). Statistical analysis using One-way ANOVA and Tukey’s post-hoc test confirmed significant differences (p < 0.05) between treatments and controls. The findings demonstrate that integrating eDNA-based biodiversity monitoring with plant-derived pesticides offers an effective and sustainable approach to pest management.
Highlights
- Organic farming systems demonstrated the highest microbial diversity, while conventional farms had the greatest pest species richness.
- Garlic, neem, and tobacco plant extracts showed concentration dependent effectiveness against Helicoverpa armigera, with neem achieving the highest mortality rate.
- Integration of eDNA biodiversity data with plant-based pest control provides a sustainable alternative to chemical pesticides.
- This study offers a novel, eco-friendly strategy for pest management and promotes resilience in agricultural ecosystems.
Introduction
Sustainable agricultural practices are essential for addressing the growing challenges of food security, environmental degradation, and biodiversity loss [1-3]. As a result, there is a growing interest in alternative pest management strategies that promote sustainability while maintaining crop productivity [1,4]. The agricultural sector faces increasing pressure to reduce its reliance on chemical pesticides, which, while effective in pest control, have significant environmental and health consequences [5]. Overuse of chemical pesticides can lead to soil degradation, water contamination, and harm to non-target species, including beneficial insects and microorganisms [6]. One promising approach to sustainable pest management is the use of plant extracts, which have been traditionally employed for their pesticidal properties [7]. Plant-based pesticides, such as neem, garlic, and tobacco extracts, offer a natural and eco-friendly alternative to synthetic chemicals. These extracts contain bioactive compounds that can effectively target pests while minimizing harm to the environment. However, while the efficacy of plant extracts in pest control has been studied in various contexts, there is limited research on their combined use with advanced monitoring techniques, such as environmental DNA (eDNA) metabarcoding [8].
eDNA metabarcoding is an innovative tool that has revolutionized biodiversity monitoring in recent years [9]. This method involves the collection and analysis of genetic material from the environment (such as soil, water, and air) to assess the presence and diversity of organisms [10]. eDNA is a non-invasive technique that allows for the identification of microbial and pest communities without the need for direct observation or trapping [11]. In agricultural systems, eDNA metabarcoding offers an unprecedented opportunity to monitor the dynamics of pest populations and microbial communities across different farming practices [12]. Recently, there has been interest in enhancing agricultural sustainability through integrating natural pest management approaches with molecular biodiversity techniques [13]. Despite the potential of eDNA as a monitoring tool, its use in the context of sustainable pest management remains underexplored [14]. The interplay between plant extract efficacy and agro-biodiversity, as measured by eDNA, has not been thoroughly investigated, leaving a significant gap in our understanding of how these methods can be combined to enhance agricultural sustainability. Particularly, it is unknown how eDNA monitoring of plant-derived pesticides affects microbial and pest communities and whether or not this integration might guide efficient pest management although promoting biodiversity.
This study aims to address this gap by evaluating the potential of eDNA metabarcoding for assessing agro-biodiversity and microbial dynamics in agricultural systems, and by exploring the impact of plant extracts on pest populations. Specifically, this research will: 1) Assess the microbial and pest diversity across different agricultural practices (organic, conventional, and agroecological), 2) Investigate the effects of plant extracts (neem, garlic, and tobacco) on pest populations, and 3) Integrate eDNA data with plant extract applications to enhance sustainable pest management strategies. By combining eDNA monitoring with plant extract-based pest control, this study seeks to provide new insights into integrated pest management systems that are both environmentally sustainable and effective.
Materials and methods
Study area and sampling sites
This study was conducted across three distinct agricultural systems in the districts of Kushtia, Jhenaidah, and Rajshahi in Bangladesh between March and May 2024, during the pre-monsoon cropping season. Each site was selected based on its unique farming practices. The organic farm in Kushtia (23°37’18.2”N 89°16’39.3”E) avoided all synthetic chemical inputs and emphasized compost and botanical pest control. The agroecological farm in Jhenaidah (23°32’14.2”N, 89°10’17.5”E) implemented integrated pest management (IPM), using biological control agents and limited chemical inputs. The conventional farm in Rajshahi (24°22’28.8”N, 88°36’52.3”E) applied synthetic fertilizers and pesticides in line with standard agronomic practices. Each farm had at least two hectares under cultivation with major crops including tomato (Solanum lycopersicum) and eggplant (Solanum melongena). Within each site, three replicate plots (10 m × 10 m) were randomly selected by using a randomized grid overlay on site maps, with a minimum 10 m buffer between plots to reduce edge effects and spatial autocorrelation. Soil, plant, and air samples were collected from each plot for comparative analysis of microbial and pest community profiles across farming systems.
Sample collection
From each of the three selected plots per farm (nine plots total), a total of 30 soil, 30 plant, and 30 air samples were collected (10 per plot), representing true biological replicates. Each plot had three sub-samples of soil, which were taken diagonally and composited to guarantee spatial coverage within plots. Soil samples were collected at a depth of 0-15 cm using a sterilized soil auger. From each plot, three sub-samples (approximately 100 g each) were taken diagonally and pooled to form one composite sample (~300 g), which was stored in sterile Whirl-Pak bags and kept on ice. Plant samples were taken from tomato and eggplant plants showing visible signs of pest infestation. Leaf and stem tissues (~5 g) were clipped with sterile scissors and placed into labeled, sterile polyethylene bags. This passive sampling approach was selected based on its proven effectiveness in capturing airborne microbes and particulates in outdoor agricultural settings [15]. The use of PBS-moistened filter paper enhances microbial capture and DNA stability. The 2-hour exposure period at 1.5 m height follows guidelines for standard bioaerosol sampling in field conditions. To validate sampling consistency, triplicate plates were used, and negative controls (unexposed plates) were processed alongside each batch to detect contamination.
Environmental DNA extraction and metabarcoding
Environmental DNA (eDNA) was extracted from soil, plant surface, and air samples using the Qiagen DNeasy PowerSoil Kit (Cat. No. 12888) following the manufacturer’s instructions [16], with bead-beating for 10 minutes to enhance cell lysis. DNA concentration and purity were assessed using a NanoDrop 2000 spectrophotometer, and integrSity was checked by 1.5% agarose gel electrophoresis. For microbial analysis, the V3–V4 region of the 16S rRNA gene was amplified using primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 785R (5′-GACTACHVGGGTATCTAATCC-3′), while pest species were identified by amplifying a 658 bp fragment of the COI gene using primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′). PCR reactions (25 µL) contained 12.5 µL of 2× Taq PCR Master Mix (Thermo Fisher Scientific), 0.5 µM of each primer, 1 µL (~10 ng) of template DNA, and nuclease-free water. Thermal cycling for 16S rRNA included initial denaturation at 95 °C for 3 min, followed by 30 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min, with a final extension at 72 °C for 5 min. For COI amplification, the same conditions were used, except the annealing temperature was adjusted to 50 °C. Amplification success was verified on 1.5% agarose gels, and only clear, single bands were selected for purification. Samples with weak or ambiguous bands were reprocessed or excluded to maintain data quality. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Cat. No. 28106) and sequenced on the Illumina MiSeq platform (2×300 bp). To ensure methodological reliability, each PCR run included both positive controls (extracted DNA from Escherichia coli and Helicoverpa armigera) and No-template Controls (NTCs) to detect cross-contamination or reagent-based artifacts. DNA extractions were conducted in a laminar flow hood using sterile tools and filter tips to avoid contamination. Each batch of 10 samples included a negative extraction control. These controls consistently showed no amplification, confirming procedural cleanliness. Sequence data were processed using QIIME2 (v2023.2). Denoising and chimera removal were performed using DADA2. Taxonomy for 16S rRNA sequences was assigned using the SILVA 138 reference database with a minimum confidence threshold of 97%, while COI sequences were identified by BLAST comparison against GenBank and BOLD databases, also using a ≥ 97% similarity threshold to ensure accurate species-level assignment.
Plant extract preparation
Three plant species, neem (Azadirachta indica), garlic (Allium sativum), and tobacco (Nicotiana tabacum), were selected based on their documented pesticidal properties. Fresh leaves of neem and tobacco and fresh garlic bulbs were collected from local sources, washed thoroughly with distilled water, and shade-dried for 5–7 days until crisp. The dried materials were ground into fine powders using a sterilized mechanical grinder. For extraction, 50 grams of each powdered plant material were soaked in 500 mL of 95% ethanol in sterile conical flasks, sealed with parafilm, and shaken on a rotary shaker at 150 rpm for 48 hours at room temperature. The mixtures were filtered through Whatman No. 1 filter paper, and the filtrates were concentrated using a rotary evaporator at 40 °C until complete solvent evaporation, confirmed by drying to a constant weight and absence of ethanol odor. The concentrated extracts were reconstituted in sterile distilled water to prepare three concentrations: 10%, 25%, and 50% (w/v). Since the extracts were fully miscible in water, no surfactant was used. All extracts were stored in sterile amber bottles at 4 °C and freshly prepared every two weeks to maintain potency.
Pest control treatment application
Pest control efficacy of the prepared plant extracts was tested in a controlled greenhouse experiment using tomato (S. lycopersicum) plants grown in 5 L plastic pots filled with sterilized soil. Plants were maintained under 25 ± 2 °C, 60% - 70% relative humidity, and a 12 h light/12 h dark photoperiod. When plants reached the 5–6 leaf stage, they were randomly assigned to treatment groups: neem, garlic, and tobacco extracts at 10%, 25%, and 50% concentrations. Each treatment had five replicate plants, and ethanol-treated plants (without extracts) served as negative controls. Treatments were applied as foliar sprays using a hand sprayer, ensuring complete leaf coverage. Sprays were applied three times at weekly intervals. To evaluate pest suppression, second-instar Helicoverpa armigera larvae were introduced at a rate of five larvae per plant immediately after the first spray. Larval survival, feeding damage (based on leaf area loss), and pest mortality were recorded at 3-, 7-, and 14-days post-application to assess the efficacy of each extract and concentration.
Microbial and Pest DNA Profiling
Environmental DNA extracted from soil, plant surfaces, and air samples was used to assess microbial and pest community composition across different agricultural practices and treatment conditions. Sequencing data obtained from Illumina MiSeq (as described in Section 2.3) were first demultiplexed and quality-filtered using the QIIME2 pipeline (version 2023.2). For bacterial community profiling, denoised 16S rRNA gene sequences were clustered into amplicon sequence variants (ASVs) using DADA2, and taxonomy was assigned using the SILVA 138 reference database with a confidence threshold of 0.97. For pest identification, COI gene sequences were aligned and compared against the GenBank and BOLD databases using BLASTn. Sequences with ≥ 97% identity to reference taxa were used for species-level identification. Relative abundance tables were generated to compare community composition across the three farming systems (organic, conventional, agroecological) and among plant extract treatments. Beta-diversity metrics (Bray-Curtis dissimilarity, UniFrac) and alpha-diversity indices (Shannon, Simpson) were calculated to explore microbial and pest diversity patterns across samples.
Statistical analysis
All statistical analyses were performed using R software (version 4.3.1) with relevant packages such as phyloseq, vegan, and ggplot2. Alpha diversity indices (Shannon, Simpson, Chao1) were calculated via estimate_richness() to assess within-sample microbial and pest diversity. Beta diversity was analyzed using Bray-Curtis dissimilarity (for abundance data) and visualized through Principal Coordinates Analysis (PCoA) to compare community composition between agricultural systems and treatments. To compare microbial and pest communities across farming systems (organic, conventional, agroecological) and plant extract treatments, PERMANOVA (adonis function in vegan) tests with 999 permutations were applied. For pest control efficacy, larval mortality, feeding damage, and survival rates were compared among treatments using one-way ANOVA followed by Tukey’s HSD post-hoc tests. A significance level of p < 0.05 was used throughout all analyses.
Results
Microbial diversity across agricultural practices
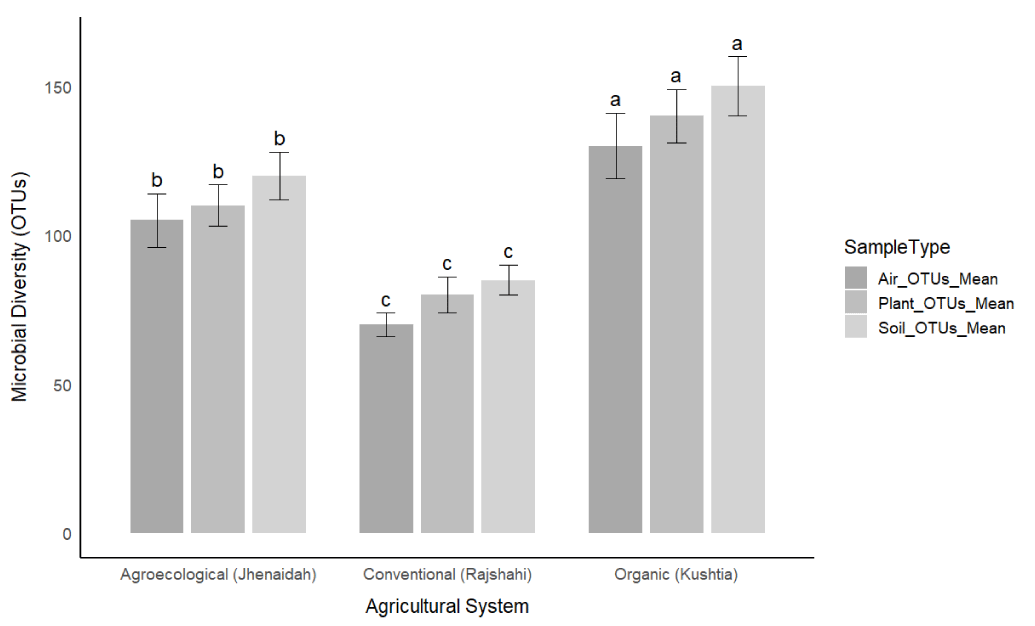
Microbial diversity was quantified in soil, plant, and air samples collected from three agricultural systems: organic (Kushtia), agroecological (Jhenaidah), and conventional (Rajshahi). Across all sample types, organic farms consistently exhibited the highest microbial diversity, followed by agroecological, with conventional farms showing the lowest diversity. Specifically, soil microbial diversity, measured as the number of Operational Taxonomic Units (OTUs), was significantly different among the systems (ANOVA, F [2,27] = 48.6, p < 0.001). Organic soils had a mean of 150 ± 10 OTUs, agroecological soils 120 ± 8 OTUs, and conventional soils 85 ± 5 OTUs (Figure 1). Similar trends were observed for microbial diversity in plant tissues (organic: 140 ± 9 OTUs; agroecological: 110 ± 7 OTUs; conventional: 80 ± 6 OTUs) and airborne microbes (organic: 130 ± 11 OTUs; agroecological: 105 ± 9 OTUs; conventional: 70 ± 4 OTUs) (Table 1). These results suggest that farming systems minimizing chemical inputs promote richer and potentially more stable microbial communities.
Pest species diversity
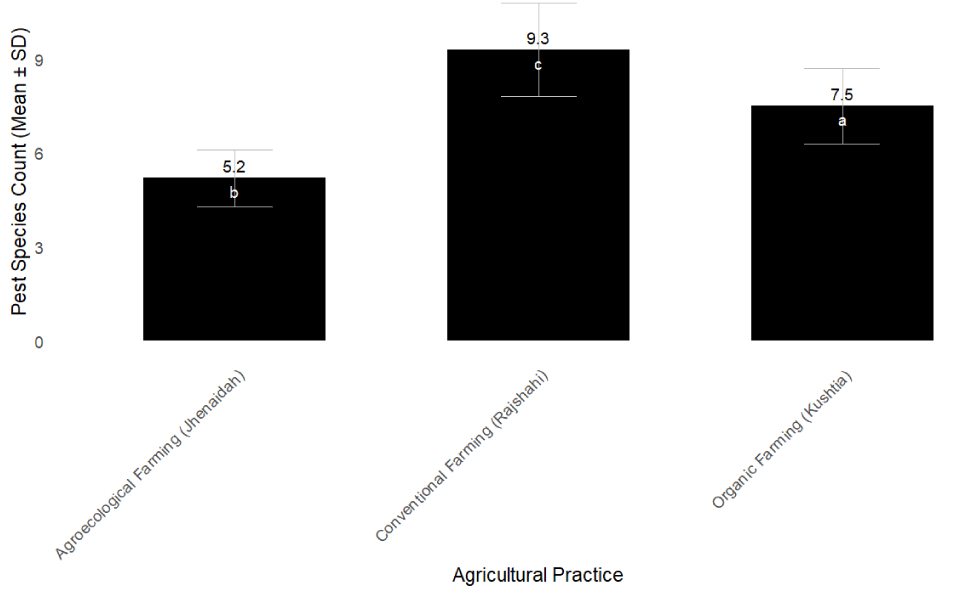
Using COI gene metabarcoding, pest species richness varied significantly across the agricultural systems (ANOVA, F [2,27] = 27.4, p < 0.001). Conventional farms harbored the greatest pest species diversity (9.3 ± 1.5 species), significantly higher than both organic (7.5 ± 1.2 species) and agroecological farms (5.2 ± 0.9 species) (Figure 2, Table 2). The lower pest diversity in agroecological farms may reflect the integrated pest management strategies employed, while the higher diversity in conventional farms could be attributed to chemical disturbance disrupting natural pest controls.
Microbial diversity in soil samples
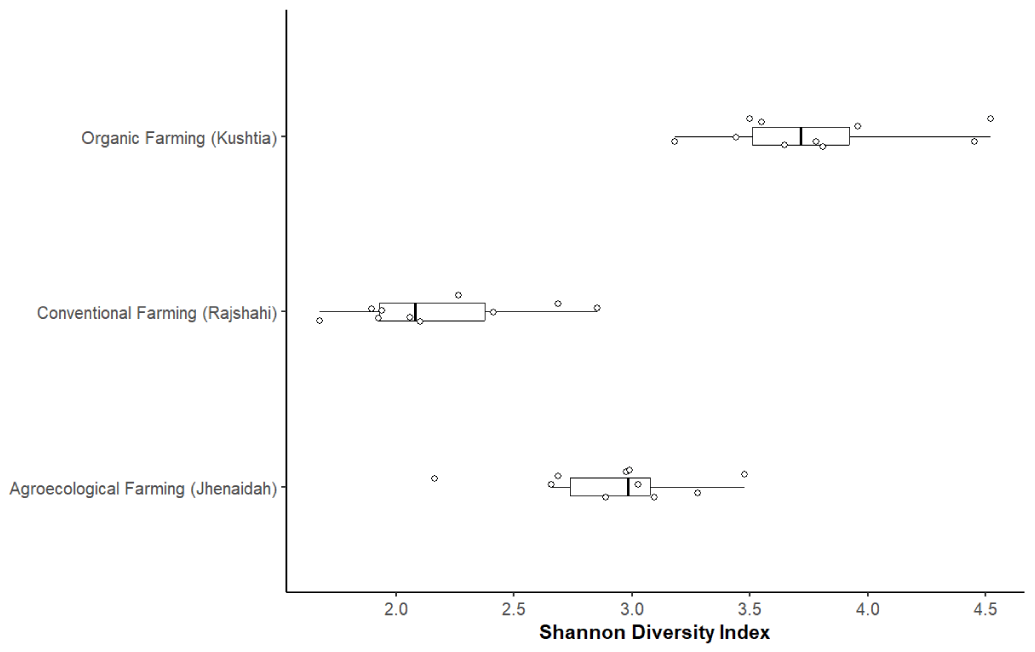
Soil microbial diversity was further characterized using Shannon diversity indices (Table 1). Organic farming soils had significantly higher Shannon diversity (3.75 ± 0.45) compared to agroecological (2.85 ± 0.35) and conventional soils (2.35 ± 0.40) (ANOVA, F [2,27] = 39.2, p < 0.001) (Figure 3). This supports the OTU-based richness data and emphasizes the positive impact of organic management on soil microbial complexity.
Effect of different plant extracts (Neem, Garlic, Tobacco) on pest reduction
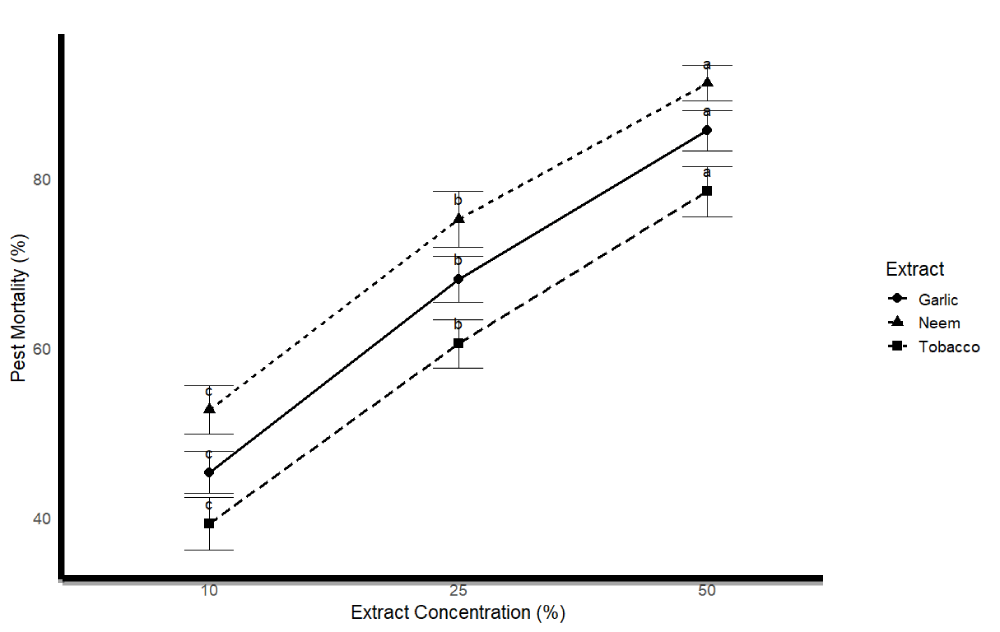
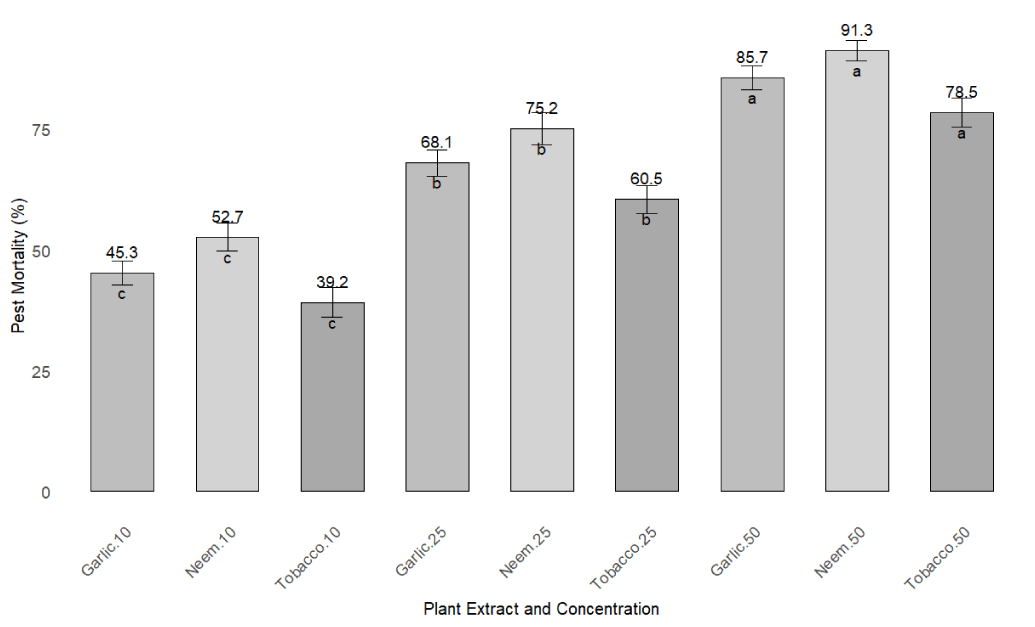
Greenhouse bioassays testing three plant extracts (neem, garlic, tobacco) against H. armigera demonstrated dose-dependent pest mortality (Figure 4). At the highest concentration (50%), neem extract induced the highest mortality (91.3% ± 2.1%), significantly exceeding garlic (85.7% ± 2.4%) and tobacco (78.5% ± 3.0%) (Figure 5) (ANOVA, F [8,36] = 25.7, p < 0.001). At 25%, neem again outperformed others with 75.2% ± 3.3% mortality. The lowest concentration (10%) yielded significantly reduced mortality across all extracts, confirming concentration-dependent efficacy. Post-hoc Tukey tests indicated that neem at 50% was significantly more effective than all other treatments (p < 0.05).
Pest mortality under different plant extract concentrations
Detailed analysis of mortality rates across concentrations revealed clear trends (Figure 5). For neem extract, mortality increased from 52.7% ± 2.9% (10%) to 91.3% ± 2.1% (50%). Garlic showed a similar trend, ranging from 45.3% ± 2.5% to 85.7% ± 2.4%, while tobacco increased from 39.2% ± 3.1% to 78.5% ± 3.0%. Differences between concentrations were statistically significant (ANOVA, p < 0.05), with 50% treatments being the most effective. These findings confirm that neem and garlic extract at higher concentrations offer promising biopesticidal activity against H. armigera.
Discussion
Our findings indicate that each agricultural system influenced the diversity and abundance of microbial communities and pest species. The organic farm in Kushtia had a moderate pest species richness, while the conventional farm in Rajshahi exhibited the highest pest diversity, likely due to the intensive use of chemical pesticides that disrupt the natural pest control mechanisms. The agroecological farm in Jhenaidah, which uses integrated pest management strategies, demonstrated the lowest pest diversity, supporting the idea that minimal pesticide use combined with ecological farming techniques could be an effective approach to maintaining pest control. Past’s decreases agricultural yields and raise management expenses, which results in significant financial losses for farmers around the world. There is a need for safer pest management options because conventional chemical pesticides, despite their effectiveness, have been connected to negative impacts on human health, non-target creatures, and environmental contamination. Microbial diversity was highest in organic system, compared to the other systems, likely due to the absence of chemical pesticides and presence of organic amendments, as a result creates favorable conditions for diver’s microbial communities. This finding aligns with previous studies demonstrating that organic farming enhances microbial abundance and functional diversity [17,18]. Conversely, the conventional farm, which relied heavily on chemical inputs, exhibited a lower microbial diversity, potentially due to the toxic effects of pesticides on beneficial microorganisms. The agroecological farm, which used a combination of organic and conventional practices, exhibited intermediate microbial diversity, indicating that a balanced approach could help maintain microbial health while ensuring pest control. The plant extract treatments, particularly neem, garlic, and tobacco, demonstrated promising results for pest control. Neem and garlic extracts showed the highest mortality rates in pests at the higher concentrations (50%), which support the previous studies demonstrating potential pesticidal properties of neem’s [19,20]. Taken together these findings offers practical implications. To improve microbial health and lessen pest pressures, policymakers, and extension services can support organic and agroecological systems. Farmers can also be advised to use plant-based pesticides, particularly garlic and neem, as efficient and environmentally responsible substrates. This directly relates to sustainable agriculture, especially in areas that aim to strike a balance between environmental stewardship and productivity. In order to validate the sustainability and effectiveness of these approaches, future studies are necessary over multiple seasons.
Conclusion
This study highlights the importance of agricultural management practices significantly shape both microbial and pest communities. Organic and agroecological farming systems promote greater microbial diversity and strong effective natural pest control, while conventional farming may lead to reduced microbial diversity and increased pest pressure. Furthermore, plant-based extracts showed excellent bio-pesticidal action against Helicoverpa armigera, particularly neem and garlic, and showed promise as substitutes for synthetic pesticides. Promoting biologically enriched systems and incorporating botanical insecticides into pest management frameworks are two practical approaches for sustainable agriculture that are suggested by these findings. Future studies should examine how these methods affect soil health, the evolution of insect resistance, and other ecosystem services over the long run. Environmental sustainability and robust food systems can both benefit from the promotion of such approaches.
Authors contributions
Abdullah Al Mamun and Shabiha Tasbir Rahman: Writing – original draft, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Abdullah Al Mamun: Supervision- supervised the whole work, and all authors approved the final manuscript.
Ethical statement
The greenhouse bioassays involving Helicoverpa armigera were conducted in accordance with institutional and national guidelines for the use of invertebrates in research. Ethical approval was not required for this study, as H. armigera is an agricultural pest species and not subject to regulation by animal ethics committees.
The authors wish to thank the agricultural biotechnology and microbial genomics laboratory and department of Biotechnology and Genetic Engineering for supporting this research.
- Çakmakçı R, Salık MA, Çakmakçı S. Assessment and principles of environmentally sustainable food and agriculture systems. Agriculture. 2023;13(5):1073. Available from: https://doi.org/10.3390/agriculture13051073
- Kabato W, Getnet GT, Sinore T, Nemeth A, Molnár Z. Towards climate-smart agriculture: Strategies for sustainable agricultural production, food security, and greenhouse gas reduction. Agronomy. 2025;15(3):565. Available from: https://doi.org/10.3390/agronomy15030565
- Al Mamun A, Rahman MM, Huq MA, Rahman MM, Rana MR, Rahman ST, et al. Phytoremediation: A transgenic perspective in omics era. Transgenic Res. 2024;33(4):175-94. Available from: https://doi.org/10.1007/s11248-024-00393-x
- Zhou W, Arcot Y, Medina RF, Bernal J, Cisneros-Zevallos L, Akbulut ME. Integrated pest management: an update on the sustainability approach to crop protection. ACS Omega. 2024;9(40):41130-41147. Available from: https://doi.org/10.1021/acsomega.4c06628
- Zhou W, Li M, Achal V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg Contam. 2024;100410. Available from: https://doi.org/10.1016/j.emcon.2024.100410
- Boonupara T, Udomkun P, Khan E, Kajitvichyanukul P. Airborne pesticides from agricultural practices: A critical review of pathways, influencing factors, and human health implications. Toxics. 2023;11(10):858. Available from: https://doi.org/10.3390/toxics1110858
- Souto AL, Sylvestre M, Tölke ED, Tavares JF, Barbosa-Filho JM, Cebrián-Torrejón G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules. 2021;26(16):4835. Available from: https://doi.org/10.3390/molecules26164835
- Milián-García Y, Pyne C, Lindsay K, Romero A, Hanner RH. Unveiling invasive insect threats to plant biodiversity: Leveraging eDNA metabarcoding and saturated salt trap solutions for biosurveillance. PLoS One. 2023;18(8):e0290036. Available from: https://doi.org/10.1371/journal.pone.0290036
- Capurso G, Carroll B, Stewart KA. Transforming marine monitoring: Using eDNA metabarcoding to improve the monitoring of the Mediterranean Marine Protected Areas network. Marine Policy. 2023;156:105807. Available from: https://doi.org/10.1016/j.marpol.2023.105807
- Bruce K, Blackman RC, Bourlat SJ, Hellström M, Bakker J, Bista I, et al. A practical guide to DNA-based methods for biodiversity assessment. Pensoft Publishers; 2021. Available from: https://doi.org/10.3897/ab.e68634
- Sivakamavalli J. Environment biomonitoring with eDNA—A new perspective to identify biodiversity. In: New Paradigms in Environmental Biomonitoring Using Plants. Elsevier; 2022. p. 109-164. Available from: https://doi.org/10.1016/B978-0-12-824351-0.00002-X
- Kestel JH, Field DL, Bateman PW, White NE, Allentoft ME, Hopkins AJM, et al. Applications of environmental DNA (eDNA) in agricultural systems: Current uses, limitations and future prospects. Sci Total Environ. 2022;847:157556. Available from: https://doi.org/10.1016/j.scitotenv.2022.157556
- Bernaola L, Holt JR. Incorporating sustainable and technological approaches in pest management of invasive arthropod species. Ann Entomol Soc Am. 2021;114(6):673-685. Available from: https://doi.org/10.1093/aesa/saab041
- Leandro C, Jay‐Robert P, Pétillon J. eDNA for monitoring and conserving terrestrial arthropods: Insights from a systematic map and barcode repositories assessments. Insect Conserv Divers. 2024;17(4):565-578. Available from: https://doi.org/10.1111/icad.12726
- Canha N, Almeida SM, Freitas MD, Wolterbeek HT. Assessment of bioaerosols in urban and rural primary schools using passive and active sampling methodologies. Arch Environ Prot. 2015;41(4):11-22. Available from: http://dx.doi.org/10.1515/aep-2015-0034
- Barcenilla C, Cobo-Díaz JF, De Filippis F, Valentino V, Cabrera Rubio R, O’Neil D, et al. Improved sampling and DNA extraction procedures for microbiome analysis in food-processing environments. Nat Protoc. 2024;19(5):1291-1310. Available from: https://doi.org/10.1038/s41596-023-00949-x
- Shu X, He J, Zhou Z, Xia L, Hu Y, Zhang Y, et al. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci Total Environ. 2022;829:154627. Available from: https://doi.org/10.1016/j.scitotenv.2022.154627
- Lori M, Symnaczik S, Mäder P, De Deyn G, Gattinger A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS One. 2017;12(7):e0180442. Available from: https://doi.org/10.1371/journal.pone.0180442
- Pretorius JC, Watt EVD. Natural products from plants: commercial prospects in terms of antimicrobial, herbicidal and bio-stimulatory activities in an integrated pest management system. In: Natural products in plant pest management. Wallingford UK: CABI; 2011;42-90. Available from: https://doi.org/10.1079/9781845936716.0042
- Lengai GM, Muthomi JW. Biopesticides and their role in sustainable agricultural production. J Biosci. 2018;6(6):7-41. Available from: https://doi.org/10.4236/jbm.2018.66002
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
