International Journal of Agricultural Science and Food Technology
Effect of Neem Oil-based Nano-emulsion Application on Nematodes Infestation and Soil Microbial Activity of Fig Seedlings
1Biology Department, College of Science and Humanities, Prince Sattam Bin Abdulaziz University, AlKharj, Kingdom of Saudi Arabia
2Pomology Department of, National Research Centre (NRC), Egypt
3Fertilization Technology Department, Agricultural and Biological Institute (NRC), Egypt
4Plant Pathology Department, National Research Center, Dokki, Cairo, Egypt
5Agricultural Microbiology Department, Agricultural and Biology Research Institute, National Research Centre, Cairo, 12622, Egypt
6Chemical Engineering and Pilot Plant Department, Engineering Institute National Research Centre, Cairo, 12622, Egypt
7Pesticides Laboratory, Agricultural Research Center, El-Sabheya, Alexandria, Egypt
8Zoology Department, Faculty of Science, Tanta University, Egypt
Author and article information
Cite this as
Alnemari AM, Mustafa NSA, El-Dahshouri MF, Noweer E MA, Elkelany US, Matter IA, et al. Effect of Neem Oil-based Nano-emulsion Application on Nematodes Infestation and Soil Microbial Activity of Fig Seedlings. Int J Agric Sc Food Technol. 2024; 10(4): 158-164. Available from: 10.17352/2455-815X.000220
Copyright License
© 2024 Alnemari AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Various chemical nematicides are currently used effectively in the control of plant parasitic nematodes, but unfortunately, they have adverse effects on humans (carcinogenic) and the environment. Neem oil is one of the safe and effective alternatives to nematicides in the soil. The effectiveness of repeated neem oil application in suppressing nematode infestation in fig seedlings was therefore examined in this study, both in its natural form (10 ml/L) and in its nano-emulsion form (5 and 7 ml/L). The number of nematodes, egg mass, and galls on the stems of infected fig seedlings were measured at that time, and the effects of these natural additives were compared with the usual chemical insecticide (Starkim). The impact of such soil amendments on some typical plant parameters, the total microbial count (CFU) of soil, and the overall enzymatic activity of the fig rhizosphere were also included. Obtained results revealed that the nematode populations were suppressed by 80.3% due to the application of nano-emulsion of neem oil (at 7 ml/L) Also, nano-emulsion of neem oil resulted in the higher reduction of nematode egg mass (60.4%) after the third application. Regarding galls, the findings were closed from 66% to 64.6, 64.3, and 64.2%, respectively, for nematicides, normal neem oils, and nano-emulsion forms). Moreover, the results for plant (water content, fresh and dry weight) and soil health parameters (total microbial count and rooting enzyme activity) were in favor of the nano-emulsion of neem oil. From the above, the results of the present work recommend the use of nano-emulsion of neem oil as one of the effective and eco-friendly means to reduce nematode infection (and many other pathogens) as a promising approach towards safe organic farming.
Agriculture output is essential to addressing the global food shortage as a result of the world’s overpopulation and climatic changes. However, agricultural crops face a wide range of challenges that include water shortages, diminishing arable land, soil degradation as a result of excessive agricultural activities, and pests and pathogens. Nematodes are common soil pests that can attack the root system and cause severe damage before any symptoms appear on the above-ground parts, especially in fertile soil. The most harmful species is the root-knot nematodes (Meloidogyne). They can cause much more damage each year ($157 billion) and this damage increases when nematode infection coincides with infection by other microbial pests [1]. Root-knot nematodes enter roots as larvae, causing lumps or nodules to form on the plant›s root system, blocking the pathways of water and nutrients transport through the plant to infect more roots [2]. Once nematodes are present in soil, they are almost impossible to eliminate, but their damage to plants can be reduced [3].
Due to the harmful effect of most chemical nematicides on humans and the environment, there is a global trend to use biological control microbes, plant extracts, and effective oils to control nematodes [4]. A lot of plants, including neem have the ability to generate secondary phytochemical metabolites useful for controlling nematodes and other parasites. The Neem plant is one of the most important natural additives used to control the spread of nematodes in the soil as well as pathogenic fungi in the soil [5]. Moreover, Javed, et al. [6] mentioned that curative application of neem formulations (i.e. oil) significantly reduced the nematodes (M. incognita) infection. Azadirachtin, the most active component I neem oil along with others like nimbin, meliantriol, nimbidin, nimbinin, salannin, nimbolides, and fatty acids (palmitic, stearic, and oleic), is responsible for its biochemical activity. Additionally, research revealed that terpinenen-4-ol, allyl isopropyl sulphide, 1,2,4-trithiolane, 3,5-diethyl, and cycloisolongifolene are some of the main active volatile components in neem essential oil [7]. Materials in its nanoform usually exhibited extraordinary properties due to their unique features, such as small dimensions and very high surface area to volume ratio [8]. The transport of bioactive compounds from plants using nanoemulsions has received a lot of interest recently. The microemulsions technique works best with bioactive essential oils that are transient and susceptible to deterioration [9].
With the environmental dimension taken into consideration, the current work aimed to investigate the direct effect of neem oil nanoemulsions on the controlling of nematode infection for fig seedlings and the influences of such applications on soil microbial activity. The effect of applying neem oil in normal and nanoemulsion form on some important parameters of the plant was also studied, in comparison with the application of the common chemical nematode Starkim.
Materials and methods
Plant material
Current work was carried out during 2023/2024 at the greenhouse of the Pomology Dept., National Research Centre, and Zoology Department, Faculty of Science, Tanta University, Egypt. About eighty uniform fig seedlings (one year old) that infected with nematodes Meloidogyne incognita) were divided into 5 groups. Each group, that contains 16 seedlings, was subjected to different treatments as the following:
Group 1: Received water
Group 2: Received nematicid (commercial product starkim) at recommended dose every month
Group 3: Received Neem oil as soil application at 10 ml/L every month
Group 4: Received Neem oil nano-emulsion as soil application at 7 ml/L every month
Group 5: Received Neem oil nano-emulsion soil as soil application at 5 ml/L every month.
Preparation of nano oil emulsion of neem
The oil used in this study was neem oil extracted from neem seeds by press cooling methods. The emulsifying agents used were tween 20 and triethanolamine of high purity grade, and finally distilled water. High shear mixer of type HAS 2003 SV25 ATORY MIXER, the HSM Series are specially designed for emulsion processes which consist of oil and water phases. At the core of each HSM unit lies the patented ‘V’ type rotor & stator in either single or double vortex design which is key in high shear mixing for efficient emulsifying or homogenizing processes. Olic acid is saponified with sodium hydroxide to produce sodium monooleate, and tween 20 is added to the produced sodium monooleate soap with a ratio of 1:1 to form the target surfactant. 750 gm of jojoba oil is mixed with 250 of the surfactant solution and mixed using a magnetic stirrer for 5 mins. The homogenized oil/surfactant solution is then subjected to a high shear mixer at 3000rpm for 15 mins. The generated mixture is added to water to the desired concentration with stirring to form the target nano oil in water emulsion.
Plant health measurements
To assess the impact of studied treatments on vegetative growth, leaf fresh and dry weight (g) were measured. Also, leaf water content was calculated, and the leaf chlorophyll content was recorded in fresh leaves by using a Minolta chlorophyll meter (SPAD – 501). In addition, leaf mineral content was determined at the end of the experiment in both seasons. Leaf samples were prepared to assess nutrient content according to Chapman and Pratt [10] as shown in Table 1.
Nematodes parameters
For evaluation of nematode reproductive parameters, the numbers of root galls and egg masses/5 g roots were recorded. The final nematode soil population was determined according to Barker [13]. The nematodes were counted and an average of eight counts was taken to determine the final population densities of M. incognita juveniles in soil and expressed as number of juveniles/250 g soil. Percentage nematode reduction was determined according to Henderson and Tilton [14]:
Nematode reduction %={1−(PTA/PTB×PCB/PCA)}×100
where PTA is the number of nematodes in the treated pots after application, PTB is the number of nematodes in the treated pots before application, PCB is the number of nematodes in the check pots before application, and PCA is the number of nematodes in the check pots after application.
Soil microbial activity: Soil samples were analyzed using the standard procedures in the laboratory at Microbial Genetics, National Research Centre (NRC).
The total microbial enzyme activities of soils were estimated based on the rate of fluorescein diacetate (FDA) hydrolytic activity according to Patle, et al. [15] with some modifications. Two grams of rhizosphere soil samples were placed (triplicates) into 50-ml capped centrifuge tubes. A volume of 15 ml potassium phosphate buffer (60 mm, pH 7.6) and 0.2 mL of 0.1% FDA (in acetone) was added to initiate the reaction. Tubes were incubated horizontally at 30 °C for 20 min in a rotary shaker. After incubation and color development, the reaction stopped by adding 15 mL of chloroform/methanol (2:1) and vortexing for one min. Tubes were subjected to centrifuge (5000 rpm for 10 min) to spindown soil and turbidity and separate chloroform layer. The developed colored fluorescein in the chloroform layer was spectrophotometrically measured at 490 nm against fluorescein standards. Total soil microbial activity was expressed as FDA hydrolysis values (µg of released fluorescein g-1 soil).
Statistical analysis
Data were analyzed as a one-way analysis of variance (ANOVA) to compare the means of multiple groups to determine if there’s a significant difference between them, and the means were represented as a combined analysis of both seasons. Data were statistically analyzed using the SAS (Statistical Analysis System) version 9.1 (Gomez and Gomez, 1984). The least significant difference (L.S.D) at 0.05 was used to compare the means of the different treatments [16] (LSD) test is a statistical method used to compare the means of multiple groups after an Analysis of Variance (ANOVA) has shown a significant difference between the groups. It helps determine which specific pairs of means are significantly different from each other.
Results and discussion
Impact of neem oil nano-emulsion on Vegetative growth of infected fig seedlings
Data in Table 2 showed that most measured vegetative parameters were increased with treatments compared with control treatment. However, these increments differed according to the type of treatment. Neem oil treatments (including nano-emulsion) showed better results compared to other treatments. Also, it was noticed that fresh weight and dry weight of leaves recorded high levels when plants were treated with neem oil at 10 ml in normal form, which validates the hypothesis that foliage growth and root growth in terms of length are better parameters to evaluate the impact of nematode infection stress [17] meanwhile water content showed the highest level when neem oil nano-emulsion was applied at 7 ml/l in comparison with other treatments, which indicated that uptake efficiency via infected root system that treated with Neem oil nano-emulsion at 7 ml/l was markedly enhanced comparing with other treatments. For chlorophyll, Table 1 indicates that all treatments enhanced chlorophyll content in the leaves of infected fig seedlings as compared with control fig seedlings. Also, there was no marked variation among treatments.
These results were supported by the obtained results of Javed, et al. [6], who reported that the application of neem formulations significantly controlled nematode infestation which positively reflected on growth performance. In addition, Norhidayah, et al. [18] showed that the application of neem extract at (25%) resulted in better performance for pre-harvest parameters of chili plants compared to the chemical pesticide.
From another angle, Usharani, et al. [19] mentioned that neem extract is considered a unique natural product for the development of biological agrochemicals (fertilizers, soil conditioners, and pesticides against various diseases) that help in improving the quality of soil, bacterial activity that is responsible for de-nitrification, hence thereby enhancing the growth of plants and fruits [20,21].
Impact of neem oil nano-emulsion on leaf nutrient content of infected fig seedlings
It was observed from data in Table 3 that infected plants (control) that received only water recorded the lowest value of minerals content meanwhile the leaf mineral content improved with other treatments starting from nematicide to neem oil treatment and neem oil nano-emulsion treatments whether 5 and 7 ml/l.
In closing view in Table 3, it was clear that neem oil surpassed nematicide in improving leaf mineral content at all levels. Also, the highest values of N P K were recorded when neem oil nano-emulsion was applied at 7m/l. In addition, micro-elements were higher with Neem oil nano-emulsion soil application at 7 ml/l.
Current results were supported by several studies whereas the findings of Javed, et al. [22] emphasized on potential of the use of neem as a biocide to manage the nematode populations and improve the growth performance of infected tomato plants. Also, the results of Saroj, et al. [23] revealed that all plant growth parameters of tomatoes improved while the nematode reproduction factors were suppressed significantly in the case of neem leaves and chemical checks as compared to untreated inoculated checks.
Impact of neem oil nano-emulsion oil on nematode parameters
The obtained data in Table 4 indicates that all treatments resulted in a decrease in Meloidogyne incognita population in treated soil compared with control. Moreover, these reduction percentages increased with the increasing number times of repeats for treatments. After, the first month of treatment application, the highest reduction was recorded with neem oil at 10 ml/l (89.4%) followed with nematicide (88.7%). Meanwhile, neem oil nano-emulsion oil treatments (5& 7 ml/l ) recorded a medium reduction percentage (65.2 & 74.5%). Also, it was observed that the reduction percentage was increased by increasing the number times of treatments repeated whereas, the reduction percentage for nematicide (starkim) increased from 88.7& to 96.1% at the 3rd time of application (3rd month) and neem oil at 10 ml/l increased from (89.4 to 90.9). For neem oil nano-emulsion (5 & 7 ml/l) the reduction percentages increased from (65.2 to 68.8%) and (74.4 to 80.35%) respectively.
The previous results strongly correspond to the results of Joymatti, et al. [24], who reported that eggs exposed to some extracts for a longer period of time decreased their rate. Although the nematicide (Starkim) was recorded with the highest reduction percentage in nematode population in the soil by increasing the number of applications (repeat), these results should not be the only essential factor to adopt this approach in controlling nematode population in the soil. The decision maker should keep in mind the effect of the treatment (nematicide) on reduction percentages in both egg mass (the means of nematode reproduction) and number of galls on infected roots. Besides, the environmental dimension should be taken into consideration which mean adopting any mechanism to control nematode infection should associated with the impact of this treatment on soil microbial count and activity. Neem oil effect according to Gommers, et al. [25], may be due to the action of the extract releasing substances into the soil which inhibits the entry of root-knot nematodes into the roots of plants.
Table 5 shows that after the first month of application, the only treatment that caused a reduction in egg mass of M. incognita was neem oil nano-emulsion at 7 ml/l which emphasized the effectiveness of neem oil nano-emulsion at 7 ml/l on decreasing the reproduction rate of nematode. With an increasing number of times of application repeats, data showed that there were considerable reduction percentages in egg mass in all treatments compared to control.
The highest reduction percentage in egg mass was recorded for neem oil nano-emulsion at 7 ml/l.
In regard to the number of galls that occurred on infected roots, data in Table 5 revealed that after the first month of applying studied treatments, only neem oil (10 ml/l) treatment caused a reduction. After three months of repeating the applications, the reduction percentages for nematicide (Starkim) (66.5%), Neem oil (10 ml/l) (64.6%), neem oil nano-emulsion (5 ml/l) (64.2%) and neem oil nano-emulsion (7 ml/l) (64.3%) were very closed, which indicates that there is no preference among tested treatments in reduction of galls on infected roots.
The obtained results in Tables 4-6 are supported by the results of several studies, Gommers, et al. [25] mentioned that neem oil may suppress nematodes penetration for roots by improving the physical properties of root barriers and improving active post-penetration bio-chemical defense which works on decreasing galls formation on infected roots which explain the considerable reduction in galls resulted by neem application that close to starkim reduction value. Also, Javed, et al. [6] showed that nematicidal metabolites of neem were absorbed by the root and they were able to disrupt the development and fecundity of nematodes that had already invaded the roots. Yasmin, et al. [26] reported that neem treatments caused a significant reduction in the population of adult females and the number of eggmasses in the soil of infected sweet gourd and that may be due to the affectivity of toxicity of neem extract on nematodes or to affectivity of neem extract in suppressing the eggs to development. A study by Sankaram, et al. [27] indicated that neem synthesizes more metabolic substances like azadirachtin and other closely related metabolites-vepaol, isovepaol, and nimibidin which have been stated to be antifeedant and growth inhibitors of insects. Such synthesized metabolites in mature seeds of neem accumulate in a more concentrated form and are likely to be more lethal to the plant pathogen including nematodes allowing better plant growth.
Soil microbial activity
In agricultural soils, rhizosphere bacteria play a key role in nutrient facilitation, the creation of plant growth stimulants, the bioremediation of toxic chemicals, and disease management. Total bacterial counts and enzyme activity are important parameters of soil quality. Both reflect the activity of the microbial population, which gave an indirect indication of soil nutrition and fertility [15].
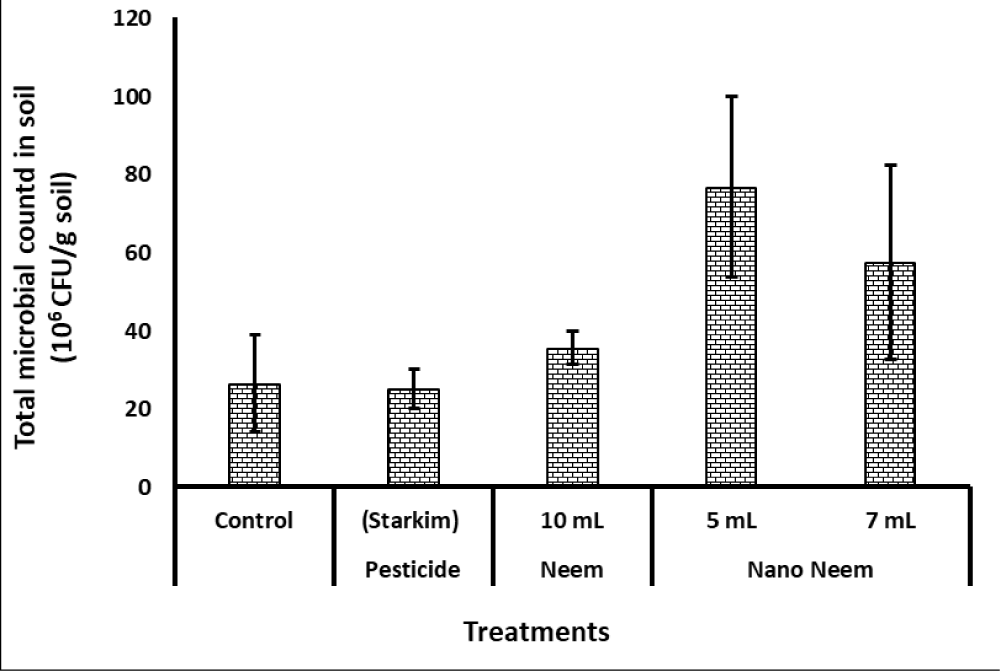
Regarding microbial counts, as presented in Figure 1, the highest microbial populations were recognized in the rhizosphere of plants treated with five- and seven mL of nano-neem. The microflora in Fig seedling’s soil treated with the commercial pesticide (Starkim) as well as that treated with 10 mL of neem oil were significantly lower than those of nano-emulsion of neem oil treated plants. However, the microbial populations of both 10-mL neem and commercial pesticide were close to the control treatment.
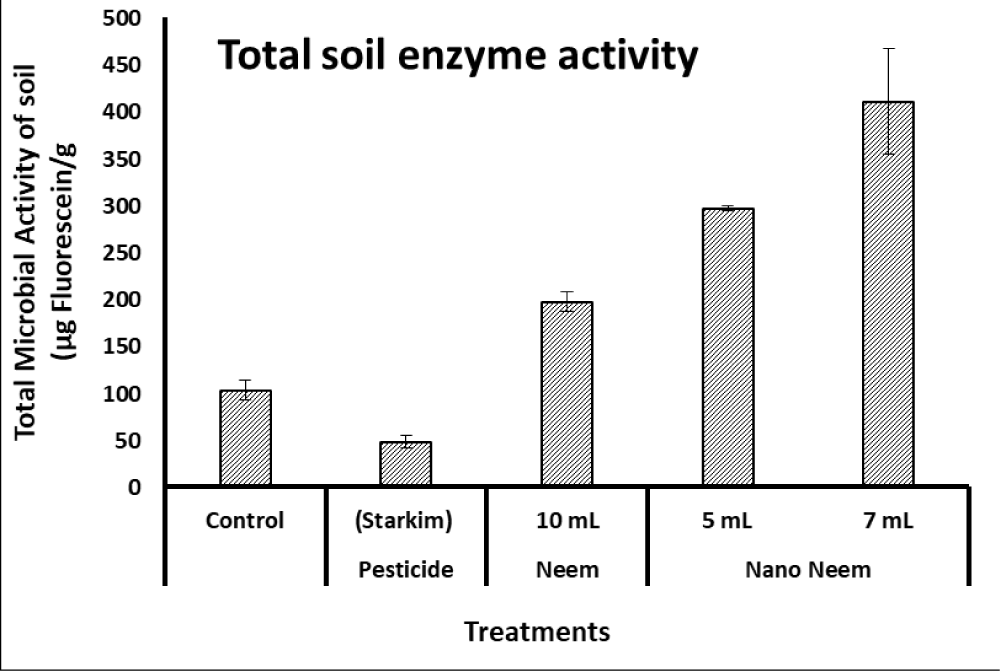
On the other hand, the enzymes produced by microbial populations in soil (such as proteases, lipases, and esterases) are capable of cleavage the colorless fluorescein diacetate into fluorescein (with a measurable fluorescent color). According to data illustrated in Figure 2, the total microbial enzyme activity of soil measured as FDA activity revealed that the 7-ml and 5-ml nano-emulsion of neem oil treatments were significantly higher than that of 10-mL neem and commercial pesticide as well as control.
The effect of soil applications on microbial counts and activity can be attributed to the direct use of nutrients that reach the soil [28]. In addition, plant nutrition and health affect the microbial activity of the soil indirectly by stimulating root exudates that contain microbial growth stimulants. These results demonstrate the positive effect of nano-emulsion of neem oil application to reduce the effect of nematode on fig plants without affecting the soil population or activity. These findings and recommendations are important in the applications of sustainable agriculture and clean environment practices that depend on the application of natural products for pest control.
Conclusion
The research endeavor sought to elucidate the efficacy of neem oil-based nano-emulsion in mitigating nematode infestations in fig seedlings while simultaneously assessing its influence on soil microbial activity. Fig seedlings were systematically categorized into five distinct groups, each subjected to varying treatments: water (control), commercial nematicide (Starkim), neem oil (10 ml/L), and neem oil nano-emulsion (5 ml/L and 7 ml/L). The investigation quantified nematode populations, egg masses, galls on stems, as well as plant health indicators (water content, fresh and dry weight), alongside soil health metrics (total microbial count and enzymatic activity). The findings indicated that neem oil nano-emulsion at a concentration of 7 ml/L effectively suppressed nematode populations by 80.3% and diminished egg masses by 60.4% following the third application. Treatments involving neem oil, particularly the nano-emulsion variant, significantly enhanced plant health parameters, including water content and both fresh and dry weight. Furthermore, the nano-emulsion treatments markedly improved soil microbial activity and total microbial count in comparison to both the control and the chemical nematicide. Ultimately, we arrived at the principal conclusions: Efficacy: Neem oil nano-emulsion demonstrates substantial effectiveness in curtailing nematode infestations and ameliorating both plant and soil health, and Environmental Sustainability: It presents a viable, eco-friendly alternative to synthetic nematicides within the framework of sustainable agricultural practices.
The authors acknowledge the National Research Centre, Giza, Egypt, for funding and providing the laboratory facilities and technical assistance.
Data availability statement
The datasets used and/or analyzed in this study are available from the corresponding author and his colleagues on reasonable request.
- Singh S, Bijendra Singh, Singh AP. Nematodes: A Threat to Sustainability of Agriculture. Procedia Environ Sci. 2015;29:215-216. Available from: https://doi.org/10.1016/j.proenv.2015.07.270
- Mai W, Abawi G. Interactions among root-knot nematodes and Fusarium wilt fungi on host plants. In: Zuckerman BM, Mai WF, Rhode RA, editors. Plant Parasitic Nematodes. Vol. II. New York: Academic Press. 1987;317-338. Available from: https://doi.org/10.1146/annurev.py.25.090187.001533
- Hajihassani AF. Chemical Nematicides for Control of Plant-Parasitic Nematodes in Georgia Vegetable Crops. UGA Cooperative Extension Bulletin 1502. 2018. Available from: https://secure.caes.uga.edu/extension/publications/files/pdf/B%201502_1.PDF .
- Osman HA, Ameen HH, Moawad M, Mohamedy R, Elkelany US. Field control of Meloidogyne incognita and root rot disease infecting eggplant using nematicide, fertilizers, and microbial agents. Egypt Biol Pest Control. 2018;28:40. Available from: https://ejbpc.springeropen.com/articles/10.1186/s41938-018-0044-1
- Kumar CV. Use of Neem Extracts (Azadirachta indica) in Control of Plant Diseases: A Review. Int J Curr Microbiol Appl Sci. 2020;Special Issue-11:3481-3487. Available from: http://www.ijcmas.com .
- Javed N, Gowen SR, Inam-ul-Haq M, Anwar SA. Protective and curative effect of neem (Azadirachta indica) formulations on the development of root-knot nematode Meloidogyne javanica in roots of tomato plants. Crop Prot. 2007;26(4):530-534. Available from: https://doi.org/10.1016/j.cropro.2006.05.003
- Mossa ATH, Mohamed RI, Mohafrash SM. Development of a ‘green’ nanoformulation of neem oil-based nanoemulsion for controlling mosquitoes in the sustainable ecosystem. Biocatal Agric Biotechnol. 2022;46:102541. Available from: http://dx.doi.org/10.1016/j.bcab.2022.102541
- Darwesh OM, Ali SS, Matter IA, Elsamahy T. Nanotextiles waste management: Controlling of release and remediation of wastes. In: Nanosensors and Nanodevices for Smart Multifunctional Textiles. Elsevier; 2021;267-286. Available from: http://dx.doi.org/10.1016/B978-0-12-820777-2.00016-9
- Ali EOM, Shakil NA, Rana VS, Sarkar DJ, Majumder S, Kaushik P, et al. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind Crops Prod. 2017;108:379-387. Available from: http://dx.doi.org/10.1016/j.indcrop.2017.06.061
- Chapman HD, Pratt PF. Methods of Analysis for Soils, Plants and Waters. University of California, Department of Agricultural Sciences. 1978;4034. Available from: https://www.scirp.org/reference/ReferencesPapers?ReferenceID=1396955
- Jackson ML. Soil Chemical Analysis. India: Prentice-Hall, Inc.; 1973.
- Lindsay WL, Norvell WA. Development of a DTPA micronutrient soil test for zinc, iron, manganese, and copper. Soil Sci Am J. 1978;42:421-428. Available from: https://doi.org/10.2136/sssaj1978.03615995004200030009x
- Barker KR. Nematode extraction and bioassay. In: Barker KR, Carter CC, Sasser JN, editors. Advanced Treatise on Meloidogyne . Vol. II. Nematology. Raleigh: North Carolina State Univ. Graphics; 1985;19-35. Available from: https://www.scirp.org/reference/referencespapers?referenceid=3622869
- Puntener W. Manual for Field Trials in Plant Protection. Basle, Switzerland: Agricultural Division, Ciba Geigy Limited; 1981;205. Available from: https://www.sciepub.com/reference/215319
- Patle PN, Navnage NP, Barange PK. Fluorescein diacetate (FDA): Measure of total microbial activity and as indicator of soil quality. Int J Curr Microbiol Appl Sci. 2018;7:2103-2107. Available from: https://doi.org/10.20546/ijcmas.2018.706.249
- Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Ames: Iowa State University Press; 1989;503. Available from: https://www.scirp.org/reference/referencespapers?referenceid=2300515
- Wareing P. Growth and its coordination in trees. In: Luckwill LC, Cutting CV, editors. Physiology of Tree Crops. Long Ashton Research Station, University of Bristol, Academic Press; 1970;1-21. Available from: https://www.scirp.org/reference/referencespapers?referenceid=582710
- Norhidayah CS, Md Yusoff NS, Lob S, Ibrahim N, Che Soh N, Mohamed J. Effect of Neem Extract on Growth Performance and Post-harvest Quality of Chili. Asian J Plant Sci. 2021;20(1):80-85. Available from: http://dx.doi.org/10.3923/ajps.2021.80.85
- Usharani KV, Naik D, Manjunatha RL. Neem as an organic plant protectant in agriculture. J Pharmacogn Phytochem. 2019;8(3):4176-4184. Available from: https://www.phytojournal.com/archives/2019/vol8issue3/PartBJ/8-2-197-547.pdf
- Smith MAK, Tolorun TP, Adeniji OS. Effect of combined mulch and fertilizer on weed growth and okra (Abelmoschus esculentus L. Moench) yield in a tropical environment. In: Proceedings of the 35th Annual Conference of the Agricultural Society of Nigeria; 2001 Sep 16-20; University of Agriculture, Abeokuta, Nigeria. p. 103-112.
- Mohanty S, Patra A, Chhonkar P. Neem (Azadirachta indica) seed kernel powder retards urease and nitrification activities in different soils at contrasting moisture and temperature regimes. Bioresearch Technol. 2008;99:894-899. Available from: https://doi.org/10.1016/j.biortech.2007.01.006
- Javed N, Anwar SA, Fayyaz S, Khan M. Effects of neem formulations applied as soil drenching on the development of Meloidogyne javanica root-knot nematode on roots of tomato. Pak J Bot. 2008;40(2):905-910. Available from: https://www.pakbs.org/pjbot/PDFs/40(2)/PJB40(2)905.pdf
- Yadav S, Patil J, Kumar A. Bio-nematicidal effect of Azadirachta indica, against Meloidogyne incognita in tomato. Int J Chem Stud. 2018;6(3):2757-2761. Available from: https://www.chemijournal.com/archives/?year=2018&vol=6&issue=3&ArticleId=2826&si=false
- Joymatti L, Dhanachand C, Devi LS. Effect of plant extracts on M. incognita . Indian J Nematol. 1998;28:225-230. Available from: https://www.indianjournals.com/ijor.aspx?target=ijor:ijn&volume=28&issue=2&article=024
- Gommers F, Barker J, Wynberg H. Dithiophenes as singlet oxygen sensitizers. Photochem Photobiol. 1982;35:615-619. Available from: https://doi.org/10.1111/j.1751-1097.1982.tb02619.x
- Yasmin L, Rashid MH, Nazim Uddin M, Hossain MS, Hossain ME, Ahmed MU. Use of neem extract in controlling root-knot nematode ( Meloidogyne javanica) of sweet-gourd. Plant Pathol J. 2003;2(3):161-168.
- Sankaram AB, Reddy VVN, Marthandamurthi M. 13C NMR spectra of some naturally occurring binaphthoquinones and related compounds. Phytochemistry. 1986;25(12):2867-2871. Available from: https://doi.org/10.1016/S0031-9422(00)83757-7
- Luziatelli F, Ficca AG, Colla G, Baldassarre Švecová E, Ruzzi M. Foliar application of vegetal-derived bioactive compounds stimulates the growth of beneficial bacteria and enhances microbiome biodiversity in lettuce. Front Plant Sci. 2019;10:60. Available from: https://doi.org/10.3389/fpls.2019.00060
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
