International Journal of Agricultural Science and Food Technology
Exogenous application of different antagonists and their secretory metabolites to manage root-knot nematodes in pea
Sana Inayat1*, Misbah Mumtaz1, Asad Ullah2, Aneesa Kaleem1 and Muhammad Amjad Ali1
2Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
Cite this as
Inayat S, Mumtaz M, Ullah A, Kaleem A, Ali MA (2024) Exogenous application of different antagonists and their secretory metabolites to manage root-knot nematodes in pea. Int J Agric Sc Food Technol 10(2): 046-052. DOI: 10.17352/2455-815X.000206Copyright License
© 2024 Inayat S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Pea (Pisum sativum L.) is a grain legume, a member of the Leguminosae family. Root-knot nematodes cause severe losses ranging from 15 to 85%. Different species of nematodes including root-knot nematodes reduce the yield of pea significantly. To control root-knot nematodes biological control is a more environment-friendly approach. The main objective of the study is to assess the effect of different antagonistic microbes and their secretory metabolites to manage root-knot nematodes in peas. Through this research, we aim to identify potential biological control agents that can be used as eco-friendly alternatives to chemical nematicides, contributing to sustainable pest management practices in agriculture. For this purpose, firstly, a brinjal seedling was transplanted for inoculum development which was inoculated with infective 2nd stage juveniles of root-knot nematodes (Meloidogyne spp.) in Department of Plant Pathology research area, University of Agriculture Faisalabad (UAF). Then two pea varieties viz. Matar sabaz and Pea-2009 were sown in pots and inoculated with nematode larvae after four weeks of sowing. Moreover, the management of nematodes was done by using the antagonists and their secretory metabolites application. The data of different plant growth and nematode-related parameters was taken and subsequently analyzed using analysis of variance and least significant difference test (LSD). Results showed that the antagonist’s treatment subsequently controlled root-knot nematodes (RKNs). Maximum plant/shoot length (47 cm), root length (25.25 cm), shoot weight (22.25 g) pod length (8.50 cm), No. of secondary shoots (8) and minimum number of galls (2.5 cluster), was observed in plants treated with Bacillus spp. (T1) while maximum No. of fruits (5.75) was observed in plants treated with Pseudomonas spp. (T2). Similarly, the number of nodules (7.50 clusters) primary shoots (5) Root weight (4.6 g) was observed in plants treated with Enterobacter spp. (T3).
Introduction
Pea (Pisum sativum L.), a native crop of Southwest Asia, is among the first crops cultivated by man [1]. It is grown in lowlands during the winter and in highlands during the summer [2]. It is a member of the Leguminosae family and a rich source of nutrients. Peas are a nutritious food in protein (27.8%), complex starches (42.65%), vitamins, minerals, dietary fibers, antioxidant components, and phosphorus. it makes a superior diet for humans. Moreover, it is also utilized as animal feed [3]. Due to its nutritional value, it is consumed and exported globally. Currently, more than 87 countries are producing dry peas in the world. The producers among them are India, Ukraine, Germany, Australia, and the United States [2].
In Pakistan, Peas are grown on almost 22.43 thousand hectares (ha) with production of 149.02 thousand tons but the average yield is 6.32 tons/ha. which is 8.20% of the total area under vegetables [4,5]. Pakistan ranks in 4th position in terms of pea production among the list of peas-producing countries [6].
Various biotic factors considerably reduce the yield of peas. Fungi and bacteria cause many diseases in peas such as Powdery mildew (Erysiphe pisi), Downy mildew (Peronospora viciae) Ascochyta blight, and bacterial blight. In addition to this different nematode species like root-knot nematodes, pea cyst nematodes, and root-lesion nematodes cause nematode diseases [7]. Root-knot nematodes especially Meloidogyne spp. have negatively impacted and emerged as a significant pest of agricultural crops [8,9]. Nematode damages diversity of host plants, such as weeds, fruits, vegetables, field crops, and field crops [10]. Root-knot nematodes (Meloidogyne spp.) are a significant class of endoparasitic plant parasites with a global distribution. They exhibit a wide host range, rapid generation, high reproductive rates, and are challenging to control due to their endoparasitic nature [11]. Root-Knot Nematodes (RKNs) are sedentary in nature with obligate parasitism in vascular tissues of plant roots. They cause root galling and root lesions, reduction of plant vigor, and rotting [12]. Moreover, fewer feeder roots with less uptake of water and nutrients are also the characteristic feature of RKNs which ultimately, results in a lack of vigor and loss of yield [9]. In the Punjab region Meloidogyne species, which cause root-knot nematodes, are significant pests of many crops, harming equally the quality and yields. But two main root-knot nematode species that cause infections are M. incognita and M. javanica [13]. Yield losses caused by root-knot nematodes vary, but in vegetables 15% - 85% yield loss [12].
Present management strategies are chemical nematicides, plant quarantines i.e., creating resistance, crop rotation, cultural practices [14], and biocontrol mediators that have been done to control nematodes. There have been efforts made to find inexpensive and risk-free substitutes to chemicals or pesticides, including the use of biological agents in plants having nematicidal and nematostatic properties. a variety of antagonists and medicinal plants that can effectively reduce root-knot nematodes have been discovered [15-17]. The purpose of this project is to evaluate the effect of different antagonists on managing root-knot-causing diseases in peas, secretory metabolites are helpful for that purpose to reduce losses. Biocontrol agents (BCAs) are commonly employed to manage various soil-borne diseases, including those caused by Root-Knot Nematodes (RKN). BCAs enhance plant resistance through several mechanisms, such as nutrient mobilization, activation of resistance-related genes, and the production of secondary metabolites like phenolics, alkaloids, saponins, and antibiotics [18]. Bacillus thuringiensis Berliner and Bacillus subtilis produce metabolites toxic to Meloidogyne spp [19]. Plant growth-promoting rhizobacteria (PGPR) such as B. subtilis confer plant resistance to pathogens by releasing phytohormones (e.g., Indole-3-acetic acid, cytokinins, gibberellins, and ethylene) and enzymes like chitinases and glucanases [20,21]. B. subtilis can Induce Systemic Resistance (ISR) in plants by producing Salicylic Acid (SA) and activating defense pathways [22,23]. Soil amendments using various organic and inorganic materials are also commonly employed. Biochar (BC), a carbon-rich material, is widely used as a plant growth enhancer. Rhizobacteria are bacteria that have a strong capability to colonize the rhizosphere [24]. Among these, aerobic endospore-forming bacteria, primarily Bacillus spp. and Pseudomonas spp., are dominant. They reside in the rhizosphere and have the ability to combat plant-parasitic nematodes (PPN) [25]. B. subtilis, commonly found in soil, has been extensively studied. Other examples of rhizobacteria that can reduce nematode populations include Serratia marsescens, Pseudomonas flourescens, Corynebacterium paurometabolu, Rhizobium etli, Bacillus mycoides, P. putida, and Stenotrophomona sp. Additionally, B. thuringiensis produces parasporal crystals during the stationary phase, which can have pesticidal properties [26].
Material and methods
Collection of infected plants for inoculum development
Okra infected with root-knot nematodes after physical identification was collected from the infected area for the development of nematode inoculum in brinjal plants. After three weeks plants were inoculated with the roots and soil infected with Meloidogyne spp. The typical agronomic practices were performed to maintain plants.
Sterilization of the soil
The sandy loam soil, consisting of silt, sand, clay, and organic matter, was mixed thoroughly. It was then air dried by spreading it on a wooden bench in a thin layer, which was covered with a plastic sheet. After that, all the materials were sieved, and the stones were removed. Formalin was used for sterilization of the soil. For this process, formalin was mixed into the soil and it was covered with a polyethene bag to prevent vapors from escaping. After seven days the polyethene bag was removed and the soil was mixed.
Inoculum development on brinjal plants
The research was done in the Department of Plant Pathology research area, University of Agriculture Faisalabad. For the development of inoculum of nematodes, Brinjal seedlings were transplanted in the pots and inoculated with 2nd stage juveniles of root-knot nematodes.
Cultivation of pea plants and inoculation with Meloidogyne spp
Two varieties of pea viz Matar Sabaz and Matar-2009 were acquired from Ayub Agricultural Research Institute (AARI), Faisalabad. After that, these peas were planted in pots in the plant pathology research area at the University of Agriculture Faisalabad, for screening. Plants of peas were transplanted into the infected soil in pots. Plants were observed for the development of symptoms due to infection of Meloidogyne spp.
Bacterial culture preparation
Different bacterial antagonists viz. Bacillus spp., Enterobacter spp., and Pseudomonas spp. were collected from the soil microbiology and biochemistry lab, ISES, University of Agriculture, Faisalabad. Bacteria were grown on Nutrient Broth (NB) to isolate pure colonies the aqueous suspension of these bacteria (105 cfu/ml) was prepared in NB media. Secretory metabolites were prepared by growing these bacteria in a culture of nutrient agar and centrifuged overnight at 250 rpm, the supernatant was collected from the culture to serve as secretory metabolites.
Management of Meloidogyne spp. different antagonists and their secretory metabolites
Different bacterial antagonists viz. Bacillus spp., Enterobacter spp., and Pseudomonas spp. were used for managing the Meloidogyne spp. The experiment was conducted in Complete Randomized Design (CRD) with five replications. Management was done by inoculation with selected antagonists and their secretory metabolites exogenous applications of antagonists were done by foliar application and soil drenching.
Data recording
Plant growth parameters, including shoot length, Root length, Number of primary shoots, Number of secondary shoots, pod length, shoot weight, and root weight were recorded after six weeks of inoculation.
Statistical analysis
The collected data were subjected to analysis of variance (ANOVA) under a completely randomized design using Statistix v. 8.1 (Steel, et al. 1997). Treatment means were compared using the least significant difference (LSD) (p ≤ 0.05).
Results
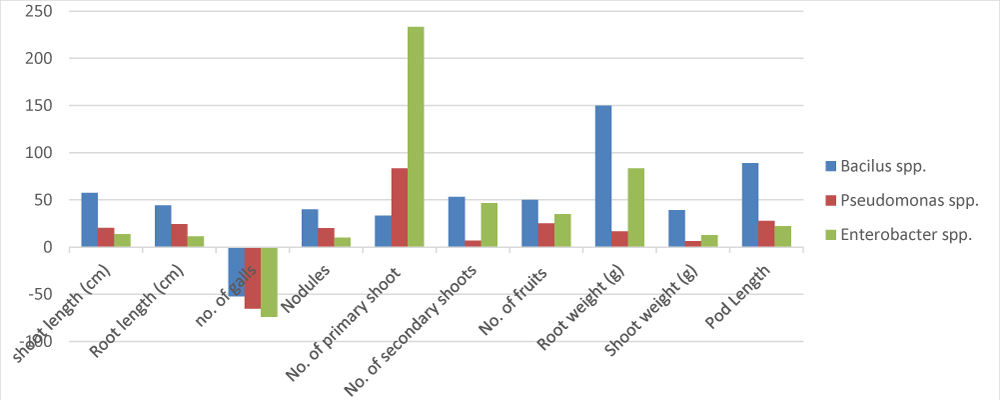
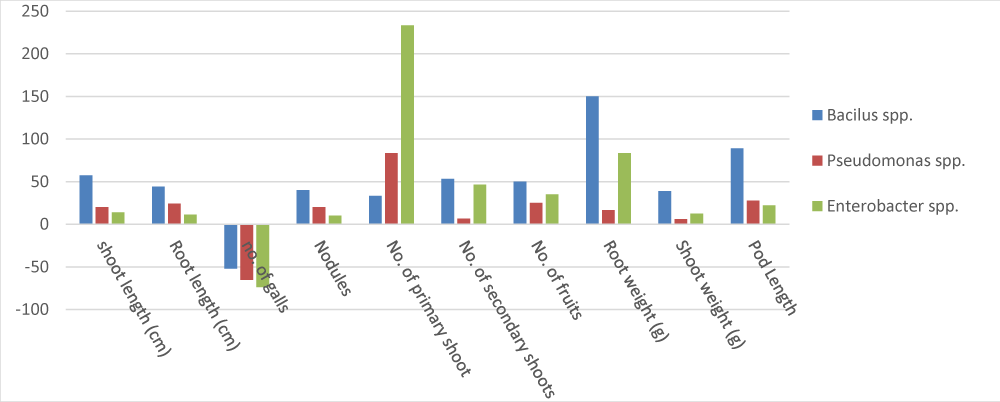
It was observed that the plants treated with treatment Bacillus spp. exhibited maximum vigor by improving overall plant growth and significantly decreased nematode infection. Meanwhile, the least shoot length was observed in control. i.e. plants only infected with M. incognita without any supplementation, in comparison with those that exhibited statistically similar shoot length. The plant growth parameters like shoot length (cm), Root length (cm), shoot weight (g), No. of secondary shoots, root weight (g), and pod length were significantly enhanced in Bacillus spp. treated pots with mean values (47 cm, 25.25 cm, 22.25, 5.75, 5 g, and 8.5 cm, respectively) as compared to Pseudomonas spp. and Enterobacter spp. However, the number of fruits (5.75) was found highest in Pseudomonas spp. treated plants. The number of primary shoots, root weight, and Nodules (cluster) were significantly increased with mean values (5, 4.6 g, and 7.5 respectively) in Enterobacter spp. treated plants. While the minimum number of galls (2.5 clusters) was also observed in Enterobacter spp. while a maximum number of galls were observed in control. In the present trial, the minimum values of the above-mentioned parameter shoot length(cm), root length (cm), number of secondary shoots, and pod length (cm) were observed in plants that receive the treatment Enterobacter spp. with mean values (26.75 cm, 16.5 cm, 3.75, 2, 1.5 and 5.5 cm respectively) (Table 1). Pseudomonas spp. treatment. Bacillus spp. showed no significant decrease in the nematode population showed somehow lowered the nematode population as compared to the control.
In the present trial, treatments (control) were applied without any bacterial treatment as shown in (Table 1). Therefore, control treatments were used to compare bacterial treatment. Apparently, the maximum number of galls decreased (-22.22) were observed in pots that were only infected with M. incognita and treated with Bacillus spp. (Table 2). However, the maximum values of parameter shoot length, root length, nodules(cluster), number of secondary shoots, and pod length increased by (70.990, 44.28, 53.33, 62.50, 150, and 88.88 respectively) were observed in plants that received the treatment Bacillus spp. followed by Pseudomonas spp. while the number of primary shoots, root weight, and mean percentage values increased by (233.333 and 53.33 respectively) were observed in plants treated with Enterobacter spp. followed by Pseudomonas spp. and Bacillus spp. (Table 2). Maximum percentage values in shoot weight increased by (43.18182) were observed in plants treated with Pseudomonas spp. followed by Bacillus spp. The maximum number of galls of nematodes were observed in control plants in which no bacterial treatment was applied. The effect of bacterial antagonist treatment on the different plant growth parameters in Matar sabaz and Pea-2009 varieties are shown in Figures 1,2. The mean square for different growth parameters and number of galls is provided in Table 3.
Discussion
Pea (Pisum sativum L.) is particularly important as an annual vegetable in cold regions [27]. Pea seeds have 23% - 25% protein content, 50% digestible starch, and 5% sugars, fibre, minerals, and vitamins [28]. M. incognita also known as the Root-Knot Nematode (RKN) is among the most common soil-borne organisms that infect a diverse selection of plant species [29]. Using biocontrol agents is essential to mitigate the soil-borne diseases caused by plant pathogens and to ensure the eco-friendly management of peas. These biological agents can inhabit the plant’s rhizosphere and effectively control diseases through various mechanisms, such as antibiosis, mycoparasitism, competition, cell wall degradation, inducing resistance, and promoting plant growth [30]. Results showed that Maximum shoot weight was observed in Bacillus spp. similar studies were also observed by [31] that the application of B. subtilis with BC increased the fresh and dry weight, in tomato plants. Masmoudi, et al. [32] also noted that treatment with Bacillus velezensis FMH2 promoted the growth of tomato plants, including improvements in root structure, plant elongation, leaf emission, fresh and dry weights. This might be due to the release of some phytohormones by the B. subtilis in the root zone i.e. Indole Acetic Acid (IAA) and Gibberellic Acid (GA3) and mobilization of essential nutrients like N, P, K+, Ca2+, and Mg2+ [33]. Wafaa, et al. 2019 [34] found that the combination of Bacillus sp. and B. subtilis significantly reduced the population of second-stage juveniles (J2) in soil, as well as the formation of galls and egg masses in roots. Additionally, the combination of Bacillus sp. and B. pumilus significantly reduced J2 in roots. When used in pairs, Bacillus spp. was more effective against M. incognita. Maulidia, et al. 2020 [35] reported that Bacillus thuringiensis AK08 effectively controlled Meloidogyne sp. by reducing galls in roots, the number of nematodes in both roots and soil, and by increasing the incubation period on tomato plants. These effects were likely due to nematotoxic compounds or extracellular hydrolytic enzymes produced by the bacteria, which destroy the nematode eggshell and juvenile cuticle. Similar studies are also observed in current findings in which the minimum number of galls were observed in Bacillus spp. This might be due to PGPR such as Bacillus sp. confers pathogen resistance to plants by releasing phytohormones (IAA, cytokinin, GA, and ethylene) as well as enzymes such as glucanases and chitinases [20,21]. Plants can develop an Induced Systemic Resistance (ISR) when Bacillus sp. produces salicylic acid (SA) and activates defense mechanisms [22,23].
Baliyan, et al. 2022 [36] reported that Bacillus cereus strain MEN8 demonstrated the ability to enhance seed germination parameters (such as germinated seeds) and stimulate plant growth, including root and shoot length, as well as the overall plant weight of chickpeas. Adeleke, et al. 2021 [37] reported that Bacillus cereus strain T4S was found to enhance sunflower growth, including taproot length, root length, root number, root weight, seed weight, and shoot weight. Kumar, et al. 2020 [38] also demonstrated that Bacillus cereus strain LPR2, both alone and in combination with silver nanoparticles (AgNPs), effectively promoted maize plant growth, encompassing root and shoot growth, as well as fresh and dry weight. This aligns with our findings because the current study also illustrated that Bacillus spp. led to an increase in pod length, shoot weight, and root length This could be due to the reason that Bacillus cereus significantly increased growth and photosynthetic ability in plants by enhancing the activity of antioxidants. This increase was evident in higher levels of proline, phytohormones, antioxidant enzymes, and improved yield parameters.
Issifu, et al. 2023 [39] demonstrate that Pseudomonas strains enhance plant growth, yield, and the presence of health-related phytochemical substances in tomato fruits. In another research, Alzate Zuluaga, et al. 2021 [40] reported that the application of bacterial consortia led to a significant increase in the number of trusses per plant. This is particularly noteworthy as the number of trusses per plant correlates directly with the number of fruits per plant. Significant differences in the number of trusses were observed at 90 and 120 Days After Inoculation (DAI). Pseudomonas monteilii (BF2P5-1) and P. fluorescens (BF4P2-5) were particularly effective in producing more trusses per plant. A higher number of trusses per plant resulted in a significant increase in the number of fruit sets (%), ultimately leading to a higher yield. This is in line with the current finding because, in the current study, Pseudomonas spp. also showed the maximum number of fruits. This might be due to the reason that Pseudomonas moraviensis has traits promoting plant growth, such as solubilizing phosphate, producing Indole-3-Acetic Acid (IAA) playing an important role in root elongation, and generating siderophores.
Bendaha, et al. 2019 [41] reported that notable enhancement in plant height, root length, and dry weight resulting in tomato plants by using Enterobacter strain EB8D inoculation led to increased nutrient absorption, especially phosphorus, as well as the production of phytohormones like Indole Acetic Acid (IAA) and siderophores. Pérez-Rodriguez, et al. 2022 [42] observed that inoculation with Enterobacter 64S1 and Pseudomonas 42P4 resulted in increased root and shoot dry weight, stem diameter, plant height, and primary shoot in tomato. In another research, Sharma, et al. 2023 [43] reported that in greenhouse experiments, it was found that inoculation with Enterobacter spp. isolate CM94 significantly increased the shoot length, root length, and fresh and dry weight of chickpea plants. Similar results were reported in the current finding in which the maximum primary shoot’s root weight was observed in Enterobacter spp. this might be due to various endophytic strains having the potential to produce substances that promote plant growth, such as Indole-3-Acetic Acid (IAA), siderophores, and phosphatase for solubilization. Indole-3-acetic acid is crucial for plant development, with L-tryptophan serving as a physiological precursor for auxin biosynthesis in both plants and microorganisms. Root exudates act as a natural source of tryptophan for rhizospheric microorganisms, enhancing the biosynthesis of phytohormones. Inoculating with PGPRs can stimulate plant growth by producing phytohormones such as indole-3-acetic acid [44].
Conclusion
The utilization of endophytic bacterium Bacillus spp., Pseudomonas spp., and Enterobacter spp. indicates its potential as a biocontrol agent against Root-knot nematodes showing positive effects on pea growth, including root weight, shoot weight, shoot length, root length, number of galls, nodules, pod length, number of secondary shoots, and number of primary shoots. These rhizobacteria offer a viable selection for enhancing soil fertility and controlling pathogens. The importance of beneficial microorganisms in suppressing soilborne plant diseases and promoting plant growth is evident.
- Zohary D. Domestication of the carob (Ceratonia siliqua L.). Israel. J. of Plant Sci. 2002; 50:141-145. DOI:10.1560/BW6B-4M9P-U2UA-C6NN
- Habib N, Zamin M. Off season pea cultivation in dir Kohistan valley. Asian. J. Plant Sci. 2003; 2(3): 283-285. DOI: 10.3923/ajps.2003.283.285.
- Bhat TA, Gupta M, Ganai MA, Ahanger RA, Bhat HA. Yield, Soil Health and Nutrient Utilization of Field Pea (Pisum sativum L.) as Affected by Phosphorus and Biofertilizers under Subtropical Conditions of Jammu. Int. J. of Mod. Plant and Anim Sci. 2013; 6(7):1-8. DOI:10.13140/RG.2.2.33954.86729.
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2016. http://faostat.fao.org/
- GOP. Fruit, Vegetables and Condiments Statistics of Pakistan. 2015-2016.Ministry of Food, Agriculture and Livestock, Govt of Pakistan, Islamabad, Pakistan. 2015.
- Kharte S, Gupta PK, Gharde Y. Exploring seed treatment and foliar application of fungicides on management of Pea diseases. Ann. Plant. Protect. Sci. 2021; 29(3): 226-230. doi: 10.5958/0974-0163.
- Grünwald NJ, Chen W, Larsen RC. Pea diseases and their management In: Diseases of Fruits and Vegetables. Vol.II, Springer, Dordrecht. 2004; 301-331. DOI:10.1007/1-4020-2607-2.
- Anwar SA, Zia A, Hussain M, Kamran M. Host suitability of selected plants to Meloidogyne incognita in the Punjab. Pak. Int. J. Nematol. 2007; 17 (2):144-150.
- Anwar SA, Mckenry MV. Incidence and reproduction of Meloidogyne incognita on vegetable crop genotypes. Pak. J. Zool.2010; 42: 135-141.
- Anwar SA, Zia A, Nazir J. Meloidogyne incognita infection of five weeds. Pak. J. Zool. 2009; 41 (2):95-100.
- Trudgill DL, Blok VC. Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol. 2001;39:53-77. doi: 10.1146/annurev.phyto.39.1.53. PMID: 11701859.
- Ali MA, Azeem F, Abbas A, Joyia FA, Li H, Dababat AA. Transgenic Strategies for Enhancement of Nematode Resistance in Plants. Front Plant Sci. 2017 May 9;8:750. doi: 10.3389/fpls.2017.00750. PMID: 28536595; PMCID: PMC5422515.
- Abd-El-Khair H, El-Nagdi Wafaa MA, Hammam MMA. Effect of olive and castor bean oil cakes singly or combined with Trichoderma spp. on Fusarium solani and Meloidogyne incognita infecting eggplant. Middle East. J. Appl Sci. 2018; 8(2):465-473.
- Chitwood DJ. Phytochemical based strategies for nematode control. Annu Rev Phytopathol. 2002;40:221-49. doi: 10.1146/annurev.phyto.40.032602.130045. Epub 2002 May 13. PMID: 12147760.
- Abo-Elyousr KA, Khan Z, El-Morsi Award M, Abedel-Moneim MF. Evaluation of plant extracts and Pseudomonas spp. for control of Root-knot nematode, Meloidogyne incognita on tomato Nematropica. 2010; 40(2): 289-299.
- Hussain MA, Mukhtar T, Kayani MZ. Assessment of damage caused by Meloidogyne incognita on pea (Abelmoschus esculentus) J. Anim. Plant Sci. 2011; 21(4):857-861.
- Hussain MA, Mukhtar T, Kayani MZ. Efficacy evaluation of Azadirachta indica, Calotropis procera, Datura stramonium and Tagetes erecta against root-knot nematodes Meloidogyne incognita. Pak. J. Bot. (Special Issue). 2011; 43:197-204.
- Janisiewicz WJ, Korsten L. Biological control of postharvest diseases of fruits. Annu Rev Phytopathol. 2002;40:411-41. doi: 10.1146/annurev.phyto.40.120401.130158. Epub 2002 Feb 20. PMID: 12147766.
- Arguelles-Arias A, Ongena M, Halimi B, Lara Y, Brans A, Joris B, Fickers P. Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb Cell Fact. 2009 Nov 26;8:63. doi: 10.1186/1475-2859-8-63. PMID: 19941639; PMCID: PMC2787494.
- Paul D, Lade H. Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain Dev. 2014; 34(4):737-752. DOI:10.1007/s13593-014-0233-6.
- Viljoen JJ, Labuschagne N, Fourie H, Sikora RA. Biological control of the root-knot nematode Meloidogyne incognita on tomatoes and carrots by plant growth-promoting rhizobacteria. Trop Plant Pathol. 2019; 44(3):284-291. DOI:10.1007/s40858-019-00283-2.
- Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res. 2018 Jan;206:131-140. doi: 10.1016/j.micres.2017.08.016. Epub 2017 Oct 17. PMID: 29146250.
- Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants--with special reference to induced systemic resistance (ISR). Microbiol Res. 2009;164(5):493-513. doi: 10.1016/j.micres.2008.08.007. Epub 2008 Oct 8. PMID: 18845426.
- Schroth MN, Hancock JG. Disease-suppressive soil and root-colonizing bacteria. Science. 1982 Jun 25;216(4553):1376-81. doi: 10.1126/science.216.4553.1376. PMID: 17798345.
- Tian B, Yang J, Zhang KQ. Bacteria used in the biological control of plant-parasitic nematodes: populations, mechanisms of action, and future prospects. FEMS Microbiol Ecol. 2007 Aug;61(2):197-213. doi: 10.1111/j.1574-6941.2007.00349.x. PMID: 17651135.
- Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998 Sep;62(3):775-806. doi: 10.1128/MMBR.62.3.775-806.1998. PMID: 9729609; PMCID: PMC98934.
- Chaudhary B, Bhatt J. Effect of different levels of inocula of Meloidogyne incognita on plant growth of Pea (Pisum sativum L.) and reproduction of nematodes. Journal of Entomology and Zoology Studies. 2020; 8(5): 2132-2135. https://doi.org/10.22271/j.ento.2020.v8.i5ac.7796
- Ram H, Hedau NK, Chaudhari GV, Kant L. Peas with zero shelling edible pods: A review. Scientia Horticulturae. 2021; 288:110333.
- Kavitha PG, Jonathan EI, Meena KS. Host-parasite relationship and pathogenicity of root knot nematode, Meloidogyne incognita in noni. Madras Agric. J. 2012; 99(10-12): 862-866.
- Junaid JM, Dar NA, Bhat TA, Bhat AH, Bhat MA. Commercial biocontrol agents and their mechanism of action in the management of plant pathogens. Int. J. Modern Plant & Anim. Sci. 2013; 1(2):39-57. https://doi.org/10.1590/S1516-8913201402540.
- Arshad U, Azeem F, Mustafa G. Combined Application of Biochar and Biocontrol Agents Enhances Plant Growth and Activates Resistance Against Meloidogyne incognita in Tomato. Gesunde P.flanzen. 2021; 73: 591-601. https://doi.org/10.1007/s10343-021-00580-4.
- Masmoudi F, Tounsi S, Dunlap CA, Trigui M. Endophytic halotolerant Bacillus velezensis FMH2 alleviates salt stress on tomato plants by improving plant growth and altering physiological and antioxidant responses. Plant Physiol Biochem. 2021 Aug;165:217-227. doi: 10.1016/j.plaphy.2021.05.025. Epub 2021 May 21. PMID: 34058513.
- Bhise KK, Dandge PB. Mitigation of salinity stress in plants using plant growth promoting bacteria. Symbiosis. 2019; 79(3):191-204. Doi: 10.1007/s13199-019-00638-y.
- Nagdi El, Wafaa MA, Abd-El-Khair H. Application of Bacillus species for controlling root-knot nematode Meloidogyne incognita in eggplant. Bull. Natl. Res. Cent. 2019; 43:154. https://doi.org/10.1186/s42269-019-0187-6.
- Maulidia V, Soesanto L, Syamsuddin S, Khairan K, Hamaguchi T, Hasegawa K, Sriwati R. Secondary metabolites produced by endophytic bacteria against the Root-Knot Nematode (Meloidogyne sp.). Biodivers. J. 2020; 21(11): 5270-5275. DOI: 10.13057/biodiv/d211130.
- Baliyan N, Dhiman S, Dheeman S. Optimization of Gibberellic Acid Production in Endophytic Bacillus cereus Using Response Surface Methodology and Its Use as Plant Growth Regulator in Chickpea. J Plant Growth Regul. 2022; 41: 3019-3029. https://doi.org/10.1007/s00344-021-10492-2.
- Adeleke BS, Ayangbenro AS, Babalola OO. Genomic Analysis of Endophytic Bacillus cereus T4S and Its Plant Growth-Promoting Traits. Plants (Basel). 2021 Aug 26;10(9):1776. doi: 10.3390/plants10091776. PMID: 34579311; PMCID: PMC8467928.
- Kumar P, Pahal V, Gupta A, Vadhan R, Chandra H, Dubey RC. Effect of silver nanoparticles and Bacillus cereus LPR2 on the growth of Zea mays. Sci Rep. 2020 Nov 23;10(1):20409. doi: 10.1038/s41598-020-77460-w. PMID: 33230192; PMCID: PMC7683560.
- Issifu M, Naitchede LHS S, Ateka EM, Onguso J, Ngumi VW. Synergistic effects of substrate inoculation with Pseudomonas strains on tomato phenology, yield, and selected human health-related phytochemical compounds. J. agro.ecosy. environ. 2023 Oct 15; 6(4) e20435. https://doi.org/10.1002/agg2.20435.
- Zuluaga MYA, Milani KML, Moreno BM, Lucini L, Valentinuzzi F, Tanja Mimmo, Youry Pii, Cesco S, Rodrigues EP, de Oliveira ALM. Inoculation with plant growth-promoting bacteria alters the rhizosphere functioning of tomato plants, Appli. Soil Ecolog. 2021 feb; 158. https://doi.org/10.1016/j.apsoil.2020.103784.
- Bendaha MEA Belaouni HA. Tomato growth and resistance promotion by Enterobacter hormaechei subsp. Steigerwaltii EB8D, Archiv.plant pathology. and plant protect. 2019 june; 52(1):1-15. DOI: 10.1080/03235408.2019.1620511.
- Pérez-Rodriguez MM, Pontin M, Piccoli P, Lobato Ureche MA, Gordillo MG, Funes-Pinter I, Cohen AC. Halotolerant native bacteria Enterobacter 64S1 and Pseudomonas 42P4 alleviate saline stress in tomato plants. Physiol Plant. 2022 Jul;174(4):e13742. doi: 10.1111/ppl.13742. PMID: 35770943.
- Sharma A, Chakdar H, Vaishnav A, Srivastava AK, Khan N, Bansal YK, Kaushik R. Multifarious Plant Growth-Promoting Rhizobacterium Enterobacter sp. CM94-Mediated Systemic Tolerance and Growth Promotion of Chickpea (Cicer arietinum L.) under Salinity Stress. Front Biosci (Landmark Ed). 2023 Oct 19;28(10):241. doi: 10.31083/j.fbl2810241. PMID: 37919081.
- Poonguzhali S, Madhaiyan M, Sa T. Isolation and identification of phosphate solubilizing bacteria from chinese cabbage and their effect on growth and phosphorus utilization of plants. J Microbiol Biotechnol. 2008 Apr;18(4):773-7. PMID: 18467875.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
