International Journal of Aquaculture and Fishery Sciences
Apparent Digestibility and Utilization of Protein and Phosphorus in diets of incorporated with Sprouted Sorghum, Phytase and Protease Enzymes for African Catfi sh (Clarias gariepinus)
1National Agricultural Research Organization (NARO) of Uganda, Mbarara ZARDI
2Department of Aquaculture and Fisheries Science, Lilongwe Universities of Agriculture and Natural Resources
3Department of Biology, Makerere University, Kampala
Author and article information
Cite this as
Kemigabo C, Kang’ombe J, Jere LW, Sikawa D, Masembe C (2017) Apparent Digestibility and Utilization of Protein and Phosphorus in diets of incorporated with Sprouted Sorghum, Phytase and Protease Enzymes for African Catfi sh (Clarias gariepinus). Int J Aquac Fish Sci. 2018; 3(4): 077-087. Available from: 10.17352/2455-8400.000033
Copyright License
© 2018 Kemigabo C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Apparent digestibility, deposition and retention of crude protein and phosphorus were determined for 30%, 35%, 50% and 55% CP diets incorporated with sprouted sorghum, 750, 1000 and 1250 unitskg-1 phytase and protease enzymes ,fed to African catfish fingerlings. All digestibility coefficients significantly differed (p<0.05) among diets with the (92.66%) observed for phosphorus in 30% and 35%CP diets with 1250 units of phytase, while that of protein (81.49%) was in 50%CP and 55% CP diets with 1250 units of protease. Crude protein deposition and retention significantly differed (p<0.05) with the highest (705.10 and 10.03 respectively) recorded in diets with 1250 protease while that of phosphorus did not in both cases (p>0.05). This demonstrated that protease was more useful in starter (high protein) diets and phytase enzyme in grower (low protein) diets and forms a basis for efficient use of phytase and protease enzymes in catfish diet formulations for different growth stages.
The North African catfish (Clarias gariepinus) is the main farmed fish species in Africa due to its fast growth and tolerance to handling and stress [1,2]. its culture is dependent on supply of quality diets which comprise about 60-70% of the operational costs. Raising catfish from fry/post hatch to table/market size requires diets with 55% -30%CP for normal growth (0.5-1kg in 6 - 8 months). Such quality diets have historically been made from fish meal. However human consumption of small pelagic fish species like silver fish used in production of fish meal reduced its supply and use in fish diets due to its high cost .This led to adoption of plant protein due to wide distribution and relatively low cost. Plant ingredients however contain antinutrients including the heat stable phytic acid that bind and reduce digestion of nutrients to support normal fish growth. Poorly digested feed pollute the environment through nutrient enrichment reducing the economic and social viability of fish farming. Unlike other antinutrients, phytic acid is found in almost all plant material [3,4]. It is not economically destroyed by a number of conventional food processing techniques including heat treatments like roasting [5]. Among techniques that unlock nutrients in plant material, sprouting is an ancient practice especially in legume and cereals. According to [6,7], sprouting activates a number of naturally occurring enzymes in plant material including phytase that exist in insoluble, low activity (210-670Ukg-1). The increase in enzymatic activity breaks down antinutrients into simple, easily absorbed nutrients. Sprouting reduced phytic acid subsequently increasing phosphorus and protein in plant fish diets and hypo-allergic baby weaning diets respectively [8]. Incorporation of sprouted sorghum into carbohydrate rich foods is also widely practiced to induce fermentation during which complex starch is reduced to simple readily assimilated glucose. To reduce processing time, most enzymes observed during sprouting have been extracted and packaged for a number of industrial processes. This not only made them expensive but also altered their efficiency because a single plant ingredient can have different antinutrients thus requiring different enzymes whose ratio in nature have not yet documented. For fish feed, enzyme sources that cope with high pelleting temperatures (>70oC), variations in diet composition and physiology of the digestive tract among species are required. Due to scanty information on digestibility of diets incorporated with sprouted grain and purified enzymes, effects of sprouted sorghum, phytase and protease enzymes on apparent digestibility of organic matter, crude protein and phosphorus was evaluated to guide efficient enzyme use.
Materials and Methods
Experimental design
Two cascading experiments of completely randomized factorial design were conducted. The first assessed the effect of dietary enzyme types and the second assessed the effect of phytase and protease levels on digestibility of catfish diets.
The first experiment was of a 4 by 4 randomized factorial design in which diets of 30%, 35%, 50% and 55% CP were each incorporated with sprouted sorghum, 750 units of phytase and protease with feed samples without enzyme as negative controls. This resulted into 16 experimental diets that were randomly assigned to 48 glass tanks in three replicates and used to assess the effect of enzyme type on digestibility. After results of the first experiment showed that apparent digestibility of diets incorporated with phytase and protease were higher than for the control and sorghum, the second experiment started to determine the optimal level of phytase and protease inclusion.
The second experiment was of a 4 by 6 randomized factorial design. Diets of 30%, 35%, 50% and 55% CP were each incorporated with 750, 1000 and 1250 units kg-1 of phytase and protease enzymes. This gave rise to 24 enzyme /diet treatments which were randomly assigned to 72 glass tanks in triplicates and used to assess the effect of enzyme level on digestibility.
Formulation of experimental diets
Diets of 30% and 35%, 50% and 55% CP were formulated using Feed Win Least Cost computer software, developed by Practical Training Center (PTC+), 1999 (currently AERES Training Centre International), Barneveld, Netherlands. For the first study, each diets was incorporated with 0.15gkg-1 of phytase enzyme (Bacillus lincheniformis 5000AUg-1 (® KOFFOZYME Israel) and 0.00125gkg-1 of protease enzyme; (Trichoderma reesei, 600,000 AUg-1 (®Cibenza, Novus international, USA) all worth 750FTUkg-1 and 10gkg-1 of sprouted sorghum flour. A portion in each case was not incorporated with any of the enzymes and served as a negative control (Table 1a). For the second experiment, phytase was incorporated at 0.2g for 1000 units and 0.25g for 1250 and protease, 0.00167 were for 1000 units and 0.00208 g for 1250 units/kg-1 (Table 1b). All the diets were incorporated with chromium (iii) oxide (Cr2O3) as inert marker at a rate of 0.05% (500g/kg1) (grade (Table 1c) [9].
The diets and enzymes were measured using a digital scale (Sartorius AG Germany, CPA52025 DS), to the nearest 0.01, mixed into water and then mixed into each diet category. The diet-enzyme mixture was left to stand for 40 minutes as incubation time and pelleted sinking 2mm diameter pellets at 56 -70oC (MUSA BODY, pelleting machine Kampala, Uganda). The pelleted diets were dried under a well-ventilated Iron roofed shade for two days. They were after packed in 2kg water proof laminated plastic bags and kept on wooden shelves at room conditions.
Stocking and management of experimental glass tanksExperimental glass tanks of 20 litre capacity were placed in doors on wooden shelves. The room was made dark by sealing off all light spaces along windows and doors using a black dump proof course (DPC) polythene paper to reduce stress associated with light sensitivity of catfish [10]. Each tank was filled with about 15ltr of dechlorinated water at room temperature (average 22oC). Water was constantly aerated using rubber air diffusers connected to an air compressor by rubber tubings. Each tank was stocked with fifteen (15) catfish fry of 10.55±2.06 gm (total length 8.25±1.34 cm). Fish was acclimatized to glass tank feeding and faecal collection by siphoning by feeding them on 2mm diameter 30% CP diet for a week. After which, experimental diets were randomly assigned to the stocked glass tanks in triplicates. The catfish fry were fed at 5% of their body weight with the daily ration divided into two portions that were fed from 10: 00 -11:00hrs and 05:00-06:00hrs daily for 60 days (2 months). Fish tanks were cleaned of leftover feed an hour after every feeding and faeces collected daily by siphoning with rubber tubing. Each time feacal matter was collected, it was placed in labeled feacal containers and kept frozen at -20oC. At the end of the experiment, all faecal samples from all the triplicate treatments were thawed and then pooled together into bigger containers. Water was constantly aerated using air blowers attached to an air compressor (HAILE ACO-328) and replaced at a rate of 50% once daily during the morning cleaning to maintain dissolved oxygen concentration between 3-4mgl-1.
Data collection
Water quality parameter: Dissolved oxygen; pH, water temperature, ammonia, reactive phosphorus (orthophosphate) and alkalinity were determined using a Sera Aqua-test kit with reagents (Sera GmbH, Heinsberg, Germany). About four (4) to six (6) drops of the appropriate reagents in the kit were mixed with 5 ml of experimental water samples. The mixture was shaken until it was evenly distributed and the color developed matched to the colour chart immediately for pH, dissolved and after five minutes for phosphates, nitrates, nitrite and ammonia. The digital value corresponding to the colour on the chart was then read and recorded under natural light.
Fish survival: Fish survival was determined by counting the number of dead fish during each cleaning and subtracting it from the original stock. Weight gain and subtracting initial weight from the final weight as; Weight gain(g)= Final weight-Initial weight.
Feed intake: The triplicate groups of fish were fed on each experimental diet to satiation twice daily from 0900-10:00hrs and 1700-1800hours at a rate of 5% of their body weight. Satiation was judged to have been reached when un eaten pellets were retained at the tank bottom. An hour after feeding ( at 10: 00hrs and 18:00hrs) the uneaten food was siphoned off the tank bottom according to [11], onto a fine sieve and gently transferred into 10ml plastic faecal containers. Care was taken to count the number of whole uneaten pellets whose weight was estimated from taking the weight of a similar number of unfed dry pellets and then subtracted from the weight of total administered feed to estimate the feed intake (Kg).
Weight gain
At the beginning and end of the experiment, the fish were fasted for 24 hours. Seven (7) samples of fish were randomly picked from each experimental treatment and their live weight measured to the nearest 0.01 g using a digital weighing scale. The difference between initial and final live weight was used as the weight gain of the fed catfish pellets from the supplied and estimating the weight of the same number of dry pellets from each respective experimental diet.
Proximate nutrient composition
At the start and end of the experiment, three fish samples from each treatment were collected and humanly sacrificed (100 mg/1 benzocaine).They were dried to constant weight and ground into fine powder (MUSA Body, Kampala Uganda). They were assessed for whole body proximate nutrient composition following standard methods by [12]. Dry matter was determined by the oven drying to constant weight (Gallencamp) SANYO,OMT OVEN, ash by muffle furnace, crude fat by ether extraction according to [13] and crude protein based on Kjeldahl’s method using a selenium catalyst, (N, 6.25) and energy (bomb calorimeter: Gallenkamp Autobomb, calibrated with benzoic acid). Chromic oxide concentration in the diets and faecal samples were determined according to [14,15].
Determination of apparent digestibility
The indirect method of determining digestibility according to (De Silva and Anderson, 1995; Guillaume et al., 2001; Jobling, 1994) was used with Chromium (iii) oxide as an inert marker at a rate of 0.5%. In all cases apparent digestibility coefficients of dry matter was calculated according to [16,17].
ADC=100-(100*% (Marker in feed/%marker in feaces))
Apparent digestibility coefficients (ADC) of crude protein and phosphorus were calculated as described by Cho,1993; De Silva and Anderson 1995 and Guillaume at al., 2001) as; ADC = I00 -[100 *(F/D) * (Di/Fi)]
Where D = % nutrient of diet; F = % nutrient of faeces; Di = % Cr2O3 in diet; Fi = % Cr2O3 in faeces according to [16,17]. Phosphorus in the diet, faeces and whole-body fish was analyzed by the molybdovanadate method as described by [18].
Determination of nutrient utilisation
Feed utilisation was judged based on the Feed conversion ratio (FCR), nutrient deposition and retention which were calculated as;
FCR= Total feed given (g)/Total weight gained (g). Phosphorus retention efficiency (PRE) was determined as; NR (%) =100*([FBW*Nf]-[IBW*Ni]/feed intake (Kg)*N diet) Where FBW is the final body weight, IBW is the initial weight; Nf is the concentration of Phosphorus in the fish at the end and Ni at the start.
Nutrient retention (NR) efficiency for phosphorus and protein were calculated as:
NR (phosphorus and crude protein (%) = 100*(FBW*Nf)-(IBW*Ni)/ (feed intake (kg)*N diet) Where FBW is the final body weight and IBW is the initial body weight of fish, N is the concentration of nutrients (phosphorus or protein) in the fish at the start (Ni) and end (Nf) of the experiment [19].
Statistical analysis
Data was entered and organized in Microsoft Excel (USA) and thereafter imported to Genstat Windows 18th Edition (AVSNI product). Data for all parameters was first analyzed for normal distribution using the Shapiro-Wilk test and homogeneity of variance using the Bartlett’s test (p < 0.001). Data in percentages/proportions was first transformed into arcsine as (ASIN (SQRT (number)) and later back-transformed by squaring the sine of the arcsine. Data that was not normality distributed even after transformations was analyzed using the non-parametric Kruskal-Wallis one-way analysis of variance (ANOVA). Data with normal distribution and homogeneity of variance was analyzed using the parametric two way analysis of variance (ANOVA). While considering interaction. Data with significant variance of the mean was subjected to pair wise comparisons using Tukey’s test. In all cases significant differences were declared at 95% confidence interval (p<0.05).
Result
Water quality
Ammonia and nitrates differed significantly among enzyme types (df = 3, p < 0.001 and H= 11.37, df=3, p= 0.010) respectively while orthophosphorus, dissolved oxygen and pH did not (H= 1.088, df =3, P= 0.780;H=1.3, df =3, P=0.729 and H= 1.307, df = 3, p=0.727 respectively). Ammonia ranged from 2.85-12.04mg/l, nitrate from 0.22-2.99mgl-1, orthophosphate from 0.37-11.41mgl-1, dissolved oxygen from 3.02-4.54 and pH from 6.48-7.45 (Table 3a).
On the basis of phytase and protease enzyme inclusion level, only ammonia significantly differed (H = 20.86, df =5, p <0.001) but nitrates, orthophosphates, dissolved oxygen and pH did not (p > 0.05) i.e. df =3, p 0.173; H = 4.508, df =3,p = 0.479; df =5,p = 0.398; df = 5, p = 0.87 respectively (Table 3b). Ammonia was highest in 50%CP diets incorporated with protease and lowest in 55%CP with phytase enzyme.
Fish survival and weight gain
Percentage survival, the weight gain and final weight (in grams) of fed catfish fry was not significantly different across the three enzyme types (H=3.789, df= 3, p = 0.151; H = 1.678, df = 3, p = 0.642 and H=1.179, df=3,p= 0.758 respectively) and the same was observed for phytase and protease inclusion levels (H=2.81,df=5, p=0.43; 3.67,df =5,p=0.60; H=5.72, df=5,p=0.33) respectively. Survival ranged from 88.89 to 100%, final weight from 33.12-39.02 and weight gain from 22.96 - 30.02g respectively.
Apparent digestibility
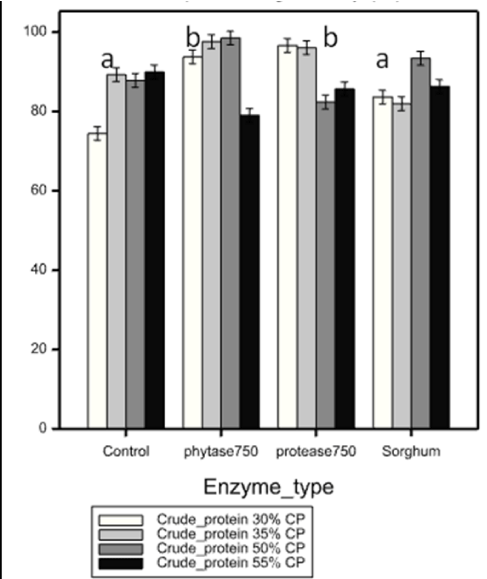
Apparent digestibility of phosphorus differed among enzyme types (df= 3, p < 0.001), being highest in diets incorporated with protease and phytase enzyme (90.15 - 92.20%) and least in the control (Figure 1).
On the other hand, digestibility of organic matter and crude protein did not differ among enzyme types (H=3.167, df =3, P= 0.367 and H = 7.511, df = 3, p = 0.057 respectively.). However, high digestibility values of organic matter (55.27-96.48%) and crude protein (71.27-96.50%) were observed in diets incorporated with phytase and protease enzymes than with sorghum and the control where 43.9 -88.95 was recorded for organic matter and 66.98 -93.77% for crude protein (Table 4a)
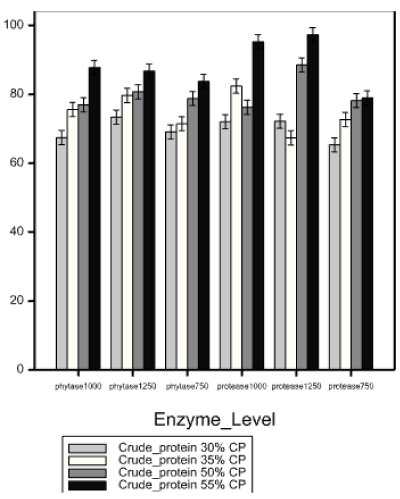
When catfish fry was fed on diets incorporated with 750, 1000 and 1250 units of phytase and protease enzyme per kilogram, apparent digestibility of organic matter, crude protein and phosphorus were significantly different (df = 5, p < 0.001; H = 11.10, df = 5, p = 0.49 and H=25.55, df =5,p <0.001) respectively. Digestibility of crude protein was highest (81.49%) in diets incorporated with 1000 units of protease (Figure 2) while that of phosphorus were highest in diets incorporated with 1250 units of phytase (Table 4b). On the other hand, apparent digestibility of organic matter was observed to be high in diets with 750 protease and least in 750 phytase (Table 4b).
Protein and phosphorus deposition
Crude protein deposition significantly differed both among enzyme types (df = 3, p= 0.004) and phytase and protease levels (H=46.39,df=5 ,p <0.001 ) but phosphorus deposition did not in either case (H = 0.773, df = 3, p = 0.856) and (H=2.59,df=5,p=0.763) respectively. Unlike phosphorus, crude protein deposition was highly influenced by diet crude protein level (CP) (df =3, p = 0.007). It varied from 242.00 -428.40 among enzyme types and increased with increasing phytase and protease activity units (242-705.10) with the highest in diets incorporated with 1250 units of protease. On the other hand, phosphorus deposition ranged from 1.20- 1.36 among enzyme types and increased (5.19-13.32) with increasing phytase activity units (Table 5).
Protein and phosphorus retention
Equally, crude protein retention differed among enzyme types (H=13.22, df = 3, p = 0.004) as well as among phytase and protease levels (H=47.5, df=5, p<0.001) while phosphorus retention did not (H = 4.752, df = 3, p = 0.191. and H=1.35, df=5, p=0.93). Retained crude protein varied from 4.12 - 6.48 among enzyme types and from 4.74-10.03 among phytase and protease levels. It was in all cases higher in diets incorporated with protease than in those with phytase and the control (Table 5). Phosphorus retention varied from 1.20 -3.16 among enzyme types and from 0.69-3.91 among phytase and protease inclusion levels (Table 6).
Result and Discussions
Water quality
The high ammonia (3.33-11.30) and phosphorus recorded in diets incorporated with protease in diets phytase (5.29-10.55 and 7.43-11.28) respectively was attributed to liberation of crude protein and phosphorus by these enzymes The ammonia and phosphorus levels were however more than torarable levels for catfish (0.2mg/l, 2mg and 0.3 mg/l (20) and allowable discharge/effluent quality of 10mgl-1 in Uganda [21]. This was attributed to accumulation of organic waste in form of disintegrated left over feed and feacal matter. However no fish deaths were registered as portrayed by high fish survival rates (88.89 to 100%). This was attributed to the high levels dissolved oxygen (3.78 - 4.32 mgl-1) that were provided by constant aeration and the morelese constant and neutral water pH (6.76 - 7.01), optimal for catfish growth (Tables 3a,3b). The observed pH stability was thought to be related to the relatively high carbonate hardness (alkalinity) of the water used (Table 3b). The high phosphorus concentrations (5.29-11.28 mgl-1) were attributed to accumulation of wastes especially uneaten feed and feaces that could not be siphoned before it disintegrated in water. Unlike in pond systems were the soil and some phytoplankton can absorb some ammonia and phosphates, no organisms (other than fish) would help in glass tanks. These water quality parameters could have negatively influenced fish growth among other factors and the actual effect was not established.
None significant differences were recorded for survival, final weight and weight gain and FCR among enzyme types or their inclusion levels. This was attributed to the influence of aeration and apparent digestibility.
The high apparent digestibility (AD) of organic matter (63.01-96.48%), crude protein (66.98-97.28) and phosphorus (74.44-98.50%) observed in diets incorporated with phytase and protease among enzyme types implied better efficiency of purified enzymes than sprouted sorghum. This could be attributed to enhanced specificity in commercial phytase and protease than in the enzyme cocktail (mix) in sprouted sorghum as reported by [6]. Efficiency of the enzyme cock tail is thought to have been degraded by proteolytic enzymes such as proteases activated by sprouting in sorghum as previously observed by [22].
Among phytase and protease levels, the high apparent digestibility of phosphorus observed in 30% and 35%CP diets incorporated with phytase enzyme especially at 1250 units per kilo was attributed to liberation of the phosphorus bound in plant ingredients that constituted 80-90% of these diets. On the other hand the high crude protein digestibility observed in 50 and 55% crude protein diets specifically at 1250 units’ kg-1 was attributed to breakdown of insoluble proteins by protease enzyme.
The apparent digestibility of organic matter and crude protein obtained were higher than 32.10-35.30% and 70.44-77.92% respectively observed by [23], when 32%CP with and without fish meal respectively were fed to Labeo rohita. Apparent digestibility of organic matter was again higher than 66.6 % for fish meal and 57.8% for soybean used as ingredients for Nile tilapia extruded diets by [24]. The high digestibility of organic matter in these diets was attributed to low crude fibre (<4) (Table 2a) Differences could have been due to incorporation of dietary enzymes, higher protein content and culture system used respectively.
Protein and phosphorus deposition and retention
High crude protein and phosphorus deposition and retention recorded in diets incorporated with protease and phytase particularly with 1250 units was ascribed to better apparent digestibility of these nutrients. Deposited crude protein to a 200-270 fold than the phosphorus deposited could have been related to higher content of crude protein in diets with 1250 protease units than in other diets. As long as energy is not limiting, increasing diet protein is known to increase protein deposition. However, retention of only 1.42-1.96% out of the crude protein deposited (242-705.10gkg-1) compared to 13.29-100% of the little phosphorus deposited indicated that the catfish was in a condition of protein balance. This implied that almost a similar amount of crude protein deposited was used up in body/metabolism and not contributing to building body tissue. This was attributed to protein breakdown to overcome effects of stress due to over handling during feacal collection. The high rate of protein breakdown was also reflected in extremely high ammonia levels, a product of protein metabolism in the culture water (Table 3a). It is in line with observations of [25], in patients with head trauma and [26], and is in agreement with [25]. On the other hand the high phosphorus retention (100%) at 750 phytase and protease inclusion levels than at 1250 units of enzymes (13.29-29.35% implied better utilisation in body tissue.
Conclusions
Incorporating 1250 units of phytase and protease enzymes in catfish diets improved digestibility and uptake of crude protein and phosphorus. We therefore recommend use of purified enzymes over sprouted sorghum until its phytase and protease activity essays and feasibility are determined.
Authors are grateful to Germany Academic Exchange Program (DAAD) and Carnegie Foundation for supporting this study through RUFORUM and government of Uganda through NARO for providing the infrastructure under which this study was conducted.
- FAO (2005-2016). National Aquaculture Sector Overview. Uganda. National Aquaculture Sector Overview Fact Sheets. Text by Mwanja, W.W. In: FAO Fisheries and Aquaculture Department Rome.
- Hecht T (2006) Regional Review on Aquaculture Development 4. Sub-Saharan Africa -2005, Rome FAO 96.
- Kumar V, Barman D, Kumar K, Mandal SC, De clercq F (2012) Anti-nutritional Factors in Plant Feedstuffs Used in Aquafeeds. World Aquaculture: 64-68. Link: https://goo.gl/HmK8xq
- Kumar V, Sinha K, Makkar HPS, De Boeck G, Becker K (2012) Phytate and phytase in fish nutrition. J Anim Physiol Anim Nutr (Berl) 96: 335-364. Link: https://goo.gl/9wZ2zn
- Kemigabo C, Kang’ombe J, Jere WL, Sikawa D, Dhikusooka TM, et al. (2017) Effect of toasting on phytic acid, protein and pH in a plant-based diet and its ingesta in fed catfish. African Journal of Rural Development 277-286. Link: https://goo.gl/sKaXFh
- Azeke MA, Egielewa SJ, Eigbogbo MU, Ihimire IG (2011) Effect of germination on the phytase activity, phytate and total phosp horus contents of rice ( Oryza sativa), maize ( Zea mays), millet ( Panicum mili aceum), sorghum ( Sorghum bicolor) and wheat ( Triticum aestivum). J Food Sci Technol 48: 724–729. Link: https://goo.gl/hvXCju
- Gupta RK, Gangoliya SS, Singh NK (2015) Reduction of phytic acid and enhancemen t of bioavailable micronutrients in food grains. Food Sci Technology 52: 676–684. Link: https://goo.gl/bVVhrg
- Sokrab AM, Ahmed IAM, Babiker EE (2012) Effect of germination on antinutritional factors, total and extractable minerals of high and low phytate corn ( Zea mays L.) genotypes. Journal of the Saudi Society of Agricultural Sciences 11: 123–128. Link: https://goo.gl/LWHsut
- Ayadi FY, Rosentrater KA, Muthukumarappan K (2012) Alternative protein sources for Aquaculture. Journal of Aquaculture Feed science and Nutrition 4: 1-26. Link: https://goo.gl/aAEYj6
- Kawamura G, Bagarinao T, Hoo PK, Justin J, Lim LS (2017) Ichthyological Research 64: 204–211.
- Ali MZ, Jauncey K (2004) Evaluation of mixed feeding schedules with respect to compensatory growth and body composition in African catfish Clarias gariepinus. Aquaculture Nutrition 10: 39–45. Link: https://goo.gl/Zeqpd5
- AOAC (2002) Official Methods of Analysis of the OAOC. Chapter 4, 1-50.
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37: 911-917. Link: https://goo.gl/a1G6FF
- Furukawa A, Tsukahara H (1999) On the acid digestion of chromic oxide as an index substance in the study of digestibility of fish feed. Bulletin of the Japanese Society of Scientific Fisheries 32: 502–506. Link: https://goo.gl/KQbjfx
- Yoo GY, Wang XJ, Choi SM, Han KM (2005) Dietary microbial phytase increased the phosphorus digestibility in juvenile Korean rockfish Sebastes schlegeli fed diets containing soybean meal. Aquaculture 243: 315–322. Link: https://goo.gl/pGW9hE
- Cho CY, Slinger SJ (1979) Apparent digestibility measurement in feedstuffs for rainbow trout. In Proc.World Symp. On finfish nutrition and fish feed technology 2: 239-247.
- Guillaume J, Kaushik S, Bergot P, Metailler R (2001) Nutrition and feeding of fish and crustaceans. Chichester,UK.: Praxis Publishing Ltd.
- Ueda I, Wada T, (1970) Determination of inorganic phosphate by the molybdovanadate method in the presence of ATP and some interfering organic bases. Anal Biochem 37: 169-174. Link: https://goo.gl/oJzzWT
- De Silva SS, Anderson TA (1995) Fish Nutrition in Aquaculture. London, England (UK). Chapman and Hall. Link: https://goo.gl/RmoCVF
- Storebakkena T, Shearerb KD, Roemc AJ (1998) Availability of protein, phosphorus and other elements in fish meal, soy-protein concentrate and phytase-treated soy-protein-concentrate-based diets to Atlantic salmon, Salmo salar. Aquaculture 161: 365–379. Link: https://goo.gl/gp8ucd
- Boyd CE, Tucker CS (1998) Pond aquaculture water quality management. Boston, MA: Kluwer Academic Publishers.
- NEMA (1999) Laws of Uganda, Chapter 153 The National Environment Act, National Environment (Standards for Discharge of Effluent into Water or on Land) Regulations, S.I. No 5. 1-5. Kampala, Uganda: National Environment Management Authority. Link: https://goo.gl/eGrkp8
- Gul Y, Salim M, Rabbani B (2007) Evaluation of apparent digestibility coefficients of different dietary protein levels with and without fish meal for labeo rohita. Pakistan Vet J 27: 121-125. Link: https://goo.gl/tXimHA
- Guimarães IG, Pezzato LE, Barros MM, Fernandes RN (2012) Apparent nutrient digestibility and mineral availability of protein-rich ingredients in extruded diets for Nile tilapia. Revista Brasileira de Zootecnia 41: 1801-1808. Link: https://goo.gl/BrDzHe
- Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, et al. (1996) Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci U S A 93: 2714-2718. Link: https://goo.gl/2BvKtJ
- Boirie Y, Gachon P, Beaufrère B (1997) Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr 65: 489-495. Link: https://goo.gl/AKSyHQ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
