International Journal of Aquaculture and Fishery Sciences
Biometry and Growth of Freshwater Turtle Kinosternon Scorpioides (chelonia: kinosternidae) on Curupu Island, Brazil
1Postgraduate Program in Biodiversity and Conservation, Federal University of Maranhão, Center for Biological and Health Sciences, 65080-805, São Luís, Maranhão, Brazil
2Department of Oceanography and Limnology, Federal University of Maranhão, Center of Biological and Health Sciences, 65080-805, São Luís, Maranhão, Brazil
3Department of Biological Sciences, Center for Biological and Health Sciences, 65080-805, São Luís, Maranhão, Brazil
Author and article information
Cite this as
Miranda Garcez RB, de Sousa Ribeiro LE, Oliveira CC, Calvet M, Barreto L. Biometry and Growth of Freshwater Turtle Kinosternon Scorpioides (chelonia: kinosternidae) on Curupu Island, Brazil. Int J Aquac Fish Sci. 2025; 11(3): 031-038. Available from: 10.17352/2455-8400.000101
Copyright License
© 2025 Miranda Garcez RB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Studies on turtle life history are often limited by their extended lifespans. This study investigated the growth patterns and biometric characteristics of Kinosternon scorpioides, a freshwater turtle species, using data collected over a 12-year period on Curupu Island, Maranhão, Brazil. Individuals were captured using traps, permanently marked for individual recognition, and standard biometric measurements—maximum carapace length, plastron length, tail length, body height, carapace width, and body mass—were recorded. Males and females exhibited similar biometric values, with the exception of tail length (greater in males) and carapace width (greater in females). The most frequent plastron length classes were 103 - 111 mm for males and 112 - 120 mm for females. While absolute plastron growth was comparable between sexes, females showed a non-significant trend of greater body mass gain. A negative relationship was observed between the exponential growth rate and the geometric mean body size in both sexes (females: R = –0.54, p < 0.0001; males: R = –0.35, p > 0.05). Growth rates did not significantly differ between sexes. These biometric and growth data likely correlate with reproductive characteristics and provide valuable insights into the ecological dynamics of K. scorpioides. Such findings are crucial for developing effective conservation strategies for the species.
Animal life history encompasses patterns of growth, differentiation, energy allocation, and reproduction [1]. These traits are thought to have coevolved in integrated suites that optimize fitness through evolutionary processes [2]. The order Testudines, distinguished by longevity and iteroparity, exhibits unique life-history strategies compared to other vertebrates [3]. Such traits not only confer effective antipredator defenses but are also intrinsically linked to body size and growth dynamics [3,4].
Research on turtle life histories remains limited, largely due to their extended lifespans which demand long-term data, often via mark-recapture methodologies [5]. Kinosternon scorpioides, a South American freshwater turtle, is widely distributed in Brazil, inhabiting states such as Pará, Maranhão, Ceará, northern Goiás, Pernambuco, and Rio Grande do Norte [6,7]. In Maranhão, populations occupy riparian floodplains and coastal lagoons [8,9]. Although primarily aquatic, K. scorpioides frequently ventures onto terrestrial habitats during breeding seasons [10] and estivates in dry periods by burying in moist substrates [4,8].
The species exhibits seasonal reproduction, with nesting from April to August and peak reproductive activity occurring between January and March [11]. Its omnivorous diet includes tadpoles, small fishes, invertebrates, algae, plant matter, and detritus [6,12]. Morphologically, K. scorpioidesis characterized by a greenish carapace with dark brown tones, cranial hinges, movable plastron lobes, chin barbels, and a tail claw [5,13]. Sexual dimorphism is pronounced, with males possessing concave plastra and longer tails facilitating copulation, while females have flat plastra [5].
Biometric traits vary considerably across turtle taxa and are essential for species or subspecies discrimination [14,15], as well as for assessing reproductive capacity and anthropogenic stress [16,17]. Kinosternids are generally small to medium sized; K. scorpioides reaches up to 270 mm carapace length [5,6]. Notably, sexual size dimorphism patterns vary, with females larger in smaller species and males often larger in bigger species [6].
Body size influences thermoregulation and is a pivotal component of life-history strategies, affecting clutch size, predator avoidance, competition, and energy reserves [1,16,18-20]. Growth trajectories are modulated by intrinsic factors such as genetic background and maternal condition [21], and extrinsic factors including temperature, food availability, and climate [22-25].
Reptiles, including turtles, generally exhibit rapid juvenile growth, often doubling their size in the first year, followed by slowed growth upon reaching sexual maturity as energy allocation shifts towards reproduction [26-28]. Mahmoud [29] observed that juveniles of several kinosternid species grow rapidly until reaching approximately 60 mm in length, after which growth decelerates. Sexual size dimorphism can influence differential growth and maturation rates between sexes [30].
In tropical populations, growth is primarily influenced by rainfall patterns and solar radiation, which respectively inhibit and promote activity and metabolic rates; temperate populations, conversely, are more affected by the length of the growing season [29]. Studies on turtle growth are valuable because of the species’ longevity and the capacity to detect shell size changes over relatively short intervals [6].
This study aims to analyze biometric parameters and elucidate the growth patterns of Kinosternon scorpioidesin a Brazilian population, thereby contributing to filling regional knowledge gaps concerning its life history.
Materials and methods
Turtles were manually captured and trapped on Curupu Island (02°24’09” to 02°27’01” S, 44°01’19” to 44°06’52” W; datum WGS 84), located approximately 30 km from downtown São Luís, in the state of Maranhão, Brazil. The island is characterized by mangroves, seasonally flooded grasslands, vegetated dunes, and freshwater ponds [9]. Due to its equatorial location, Curupu Island experiences intense climatic variability, which allows for a clear division between two seasons: a rainy season (December to June) and a dry season (July to November) [31,32]. The lagoons, filled by rainwater during the wet season, can reach depths of up to 1.80 m, and may completely dry out during the dry season [9].
Sampling was conducted every 15 days with two-day field stays on the island, from 2001 to 2013, through the continuous efforts of the Queamar Project (Chelonians of Maranhão), totaling 12 years of field data. We used funnel-shaped traps made of wooden stakes and mesh, approximately 1 meter in length, similar to those used for lobster capture. Fish bait was used to attract the turtles. Traps were placed in six freshwater ponds of varying sizes (approximately 5 - 50 m in width).
Biometric measurements were obtained using a caliper with 1 mm precision, and body mass was measured with a digital scale accurate to 10 g. Individuals were permanently marked using a notching system on the marginal scutes of the carapace, following the coding method [33-37]. Sex was determined based on tail length and plastron concavity, as described by Trebbau and Pritchard [6].
We recorded the following biometric parameters for each individual: maximum carapace length (CL), maximum carapace width (CW), maximum plastron length (PL), maximum body height (BH), maximum tail length (TL), and body mass (W). Data analyses were based on a subset of records from the Queamar Project database, collected between 2001 and 2013.
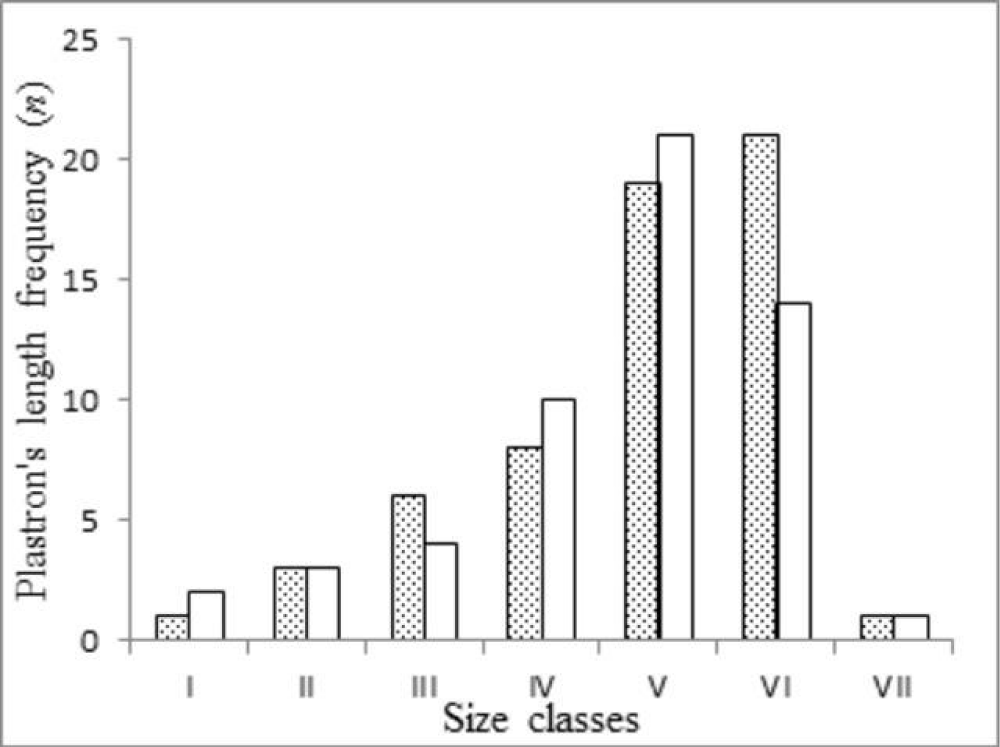
To describe the biometric characteristics of Kinosternon scorpioides, we calculated the mean values and standard deviations for each parameter by sex. Differences between males and females were assessed using analysis of variance (ANOVA). Individuals were grouped into size classes to evaluate variation in body mass and other size-related traits. Size classes were defined based on the method of Vazzoler [38], which calculates the range between the smallest and largest observed plastron lengths. The number of classes (Nc) was determined using Sturges’ formula [39]: Nc = 1 + (3.3 × log n), where n is the total sample size. This resulted in seven size classes, each comprising a 7 mm interval: I (66–74 mm), II (75–83 mm), III (84–93 mm), IV (94–102 mm), V (103–111 mm), VI (112–120 mm), and VII (121–129 mm).
We calculated absolute annual plastron growth (G; mm/year) using the formula:
G = (PL_recapt − PL_capt) / ΔT, where PL_recapt and PL_capt are plastron lengths at recapture and initial capture, respectively, and ΔT is the time interval between captures in years. Similarly, annual body mass gain (g/year) was calculated. Differences in growth and mass gain between sexes were tested using ANOVA.
Exponential growth rate (EG) was calculated as: EG = (log PL_recapt − log PL_capt) / ΔT, and compared between sexes using ANOVA. Additionally, to evaluate the influence of body size on growth, we performed simple linear regressions of EG against the geometric mean size (GS), calculated as: GS = √(PL_recapt × PL_capt), to minimize bias from variation in recapture intervals [40].
Statistical analyses were conducted using Statistica 10 [41]. We verified model assumptions by inspecting residual plots for normality and homoscedasticity. Where appropriate, analysis of covariance (ANCOVA) was used to control for the effect of body size (GS or CL) when comparing growth rates or biometric variables between sexes, allowing us to adjust for size-related variation and isolate the effect of sex on the parameters evaluated.
Results
A total of 929 individuals of Kinosternon scorpioides were recorded on Curupu Island. After excluding turtles that were never recaptured, 507 capture-recapture events remained, representing 113 marked individuals—54 males (208 records) and 59 females (234 records). For growth analyses, we excluded records with recapture intervals shorter than 12 months. As a result, 34 females and 26 males were included in the growth dataset.
The mean carapace length (CL) was 119.0 ± 11.4 mm for females and 116.0 ± 10.6 mm for males (mean ± SD). ANOVA results indicated no significant difference in CL between sexes (F = 3.78, p > 0.05). However, tail length (TL; p = 0.0001) and carapace width (CW; p = 0.04) differed significantly between sexes. These differences remained significant when controlling for carapace length using ANCOVA (TL: F = 15.42, p < 0.001; CW: F = 4.23, p = 0.042). Overall, biometric traits were similar between sexes, except for TL, which was consistently shorter in females (Table 1). Males were most frequently observed in size classes V and VI, while females were more evenly distributed across all classes (Figure 1).
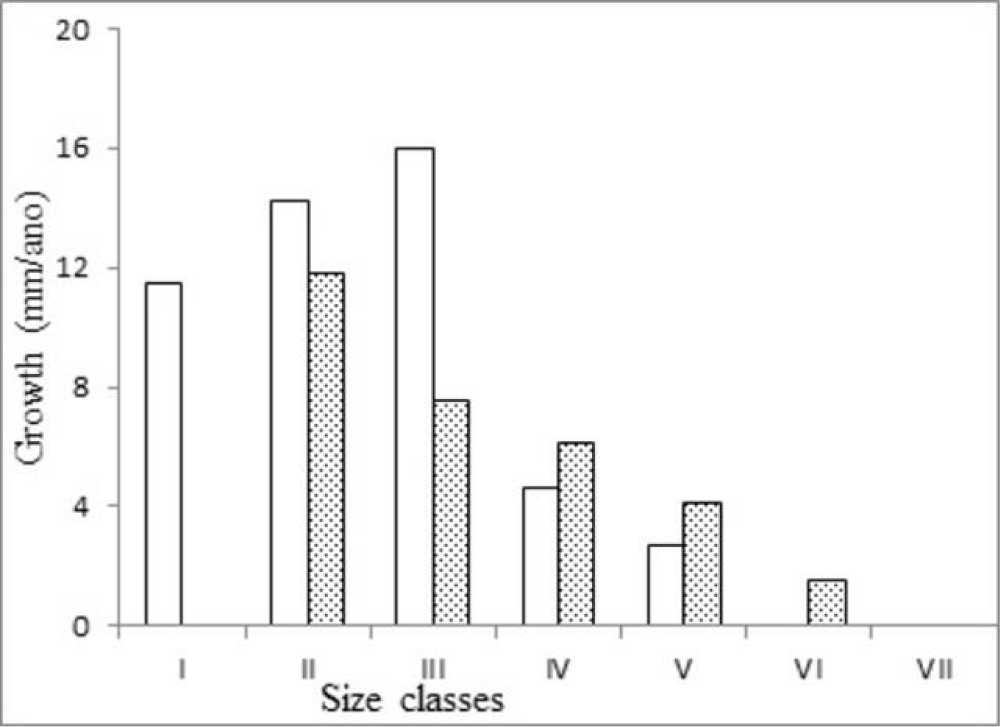
The absolute plastron growth rate (mm/year) was similar between sexes, and females exhibited slightly higher mean body mass gain (g/year) (Table 2). ANOVA results showed no significant differences in either growth metric between sexes (body mass gain: F = 0.73, p > 0.05; plastron growth: F = 0.04, p > 0.05). ANCOVA analyses confirmed these findings after adjusting for geometric mean size (GS), with no significant effect of sex on either body mass gain (F = 1.12, p = 0.29) or plastron growth (F = 0.85, p = 0.36).
In the smaller size classes (I to III), males exhibited higher average growth rates than females. From size class IV onward, females showed greater growth. Among females, growth rates declined progressively with increasing body size, indicating a consistent allometric trend (Figure 2).
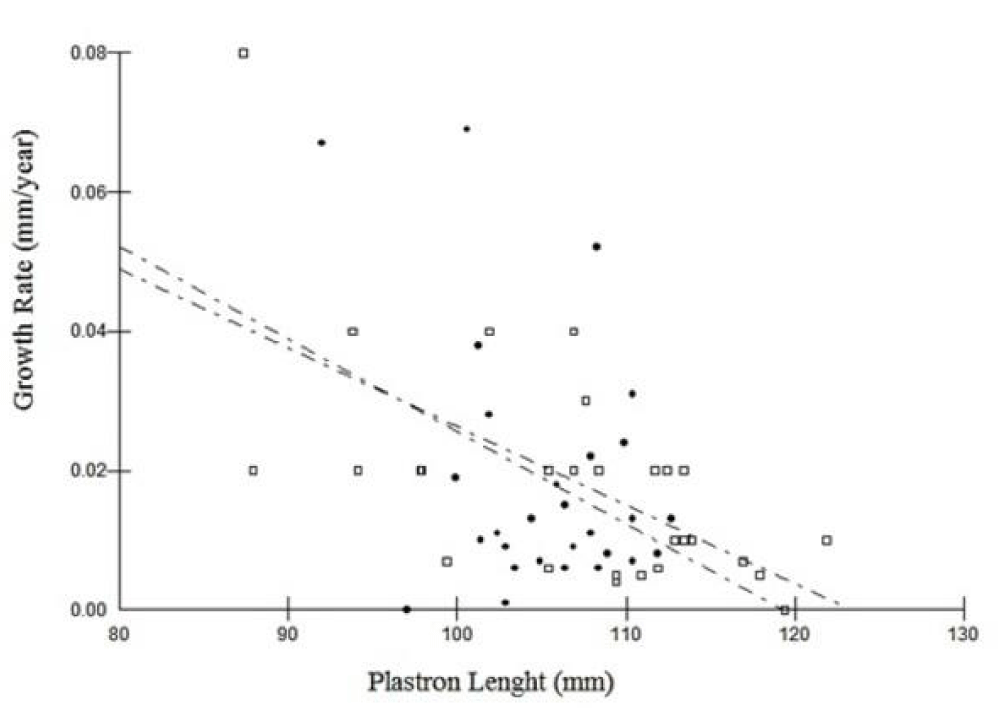
Linear regression revealed a significant negative relationship between exponential growth rate (EG) and geometric mean size (GS) in females (R = –0.54, slope = –0.28, p = 0.00007), suggesting reduced growth with increasing body size. Males exhibited a similar but non-significant trend (R = –0.35, slope = –0.12, p = 0.06) (Figure 3). ANCOVA indicated no significant interaction between sex and body size on EG (F = 0.48, p = 0.49), and no significant difference in EG between sexes after adjusting for GS (F = 0.37, p = 0.50). Model residuals met the assumptions of normality and homoscedasticity in all linear models.
Discussion
The species Kinosternon scorpioides can reach a maximum carapace length of 270 mm [6]. However, individuals in this study were considerably smaller, consistent with previous findings in Maranhão, where maximum carapace lengths reached up to 139 mm [9,42]. On Curupu Island specifically, recent studies in Maranhão reported females reaching up to 154 mm and males up to 165 mm in carapace length [42,43]. These size ranges align broadly with reports from Venezuela, French Guiana, Costa Rica, and Mexico [6,44,45].
Our first hypothesis posited that individuals in equatorial regions might exhibit smaller adult sizes compared to populations at higher latitudes. Although this study did not directly measure environmental variables, the observed smaller mean body sizes on Curupu Island relative to other regions may be consistent with latitudinal variation in growth potential influenced by climatic factors such as temperature and solar radiation. Future studies measuring these abiotic factors are necessary to test their specific effects on growth and size in K. scorpioides.
The second hypothesis addressed the potential influence of local ecological pressures on growth and reproductive investment. We propose that factors such as interspecific competition with Trachemys adiutrix and higher mortality among smaller individuals may impose energetic constraints, potentially delaying reproductive investment and limiting growth. This is supported by Medeiros, et al. [43] report of delayed sexual maturity in females from Maranhão, although direct measurements of competition or mortality rates were beyond the scope of this study.
Biometric analyses revealed significant sexual dimorphism in tail length, with males exhibiting longer tails, confirming previous observations [5]. Additionally, females showed wider carapaces, a trait possibly linked to increased reproductive capacity through greater energy storage and egg production [3,30,46-48]. These findings support the hypothesis that sexually dimorphic traits in K. scorpioides reflect divergent selective pressures related to reproduction and mobility [42].
Our data indicated a broad distribution of size classes, including larger individuals, suggesting successful adult survival across multiple years—a pattern consistent with the life-history traits typical of turtles characterized by high adult survivorship and elevated juvenile mortality [30,49]. The predominance of larger individuals in intermediate size classes may also relate to behavioral or capture biases, warranting further behavioral ecological research.
Although females tended to gain more body mass annually than males, this difference was not statistically significant. This observation aligns with the expectation that female energy allocation may prioritize reproductive structures; however, detailed physiological or reproductive data are needed to confirm this.
The growth pattern identified—declining growth rates with increasing body size—is typical for turtles [50,51]. This trend likely reflects the shift of energy allocation from somatic growth to reproduction following sexual maturity [50,51]. The correspondence between the size at which growth rate declined and previously reported female maturity thresholds [42] further supports this interpretation.
Sex-specific growth rates did not differ significantly, consistent with observations in related species such as K. subrubrum [29]. However, males exhibited higher growth rates in smaller size classes, potentially reflecting a life-history strategy favoring early maturation and reduced vulnerability in competitive interactions [52]. These dynamics highlight the importance of considering ontogenetic shifts in growth patterns in future studies.
From a conservation and management perspective, the demographic traits observed—slow growth, delayed maturity, and longevity—underline the species’ vulnerability to anthropogenic impacts and environmental changes [53]. Given that K. scorpioides is heavily exploited in the Brazilian Amazon [54,55], and considering its life-history constraints, management strategies should emphasize protection of juvenile and subadult cohorts to enhance population resilience.
Specifically, conservation efforts should prioritize: (1) Habitat preservation and restoration of freshwater ecosystems critical for all life stages, including breeding and foraging habitats; (2) Regulation of harvest to reduce unsustainable exploitation, coupled with community education and enforcement; (3) Development of captive breeding and head-starting programs to improve juvenile survival, supported by monitoring to assess program effectiveness; (4) Further ecological research focused on species interactions, reproductive biology, and growth dynamics to inform adaptive management.
Incidents such as the fire that killed approximately 100 individuals during estivation highlight the urgent need for fire management and local engagement to mitigate anthropogenic threats. Additionally, ongoing deforestation in the region [56] demands integrated land-use policies that balance human development with biodiversity conservation.
This study provides essential baseline data on size and growth parameters critical for population modeling and management planning. We advocate for multi-faceted conservation approaches that combine habitat protection, sustainable use, and targeted research to ensure the long-term persistence of K. scorpioidespopulations on Curupu Island and beyond. By analyzing biometric and growth data over multiple years, we identified size-related growth patterns, confirmed key sexually dimorphic traits, and demonstrated the absence of significant sex-based differences in growth rates. These findings fill a critical gap in the understanding of growth patterns for K. scorpioidesin equatorial regions, where data from wild populations have been scarce and often limited to descriptive accounts.
To strengthen conservation and management strategies for this species, future research should incorporate complementary approaches such as: (1) Telemetry studies to track movement patterns, habitat use, and seasonal behaviors; (2) Long-term reproductive monitoring to evaluate clutch frequency, nesting success, and age at maturity; (3) Habitat quality assessments, including monitoring of water bodies and vegetation structure, to detect environmental changes that may influence growth and survival; (4) Mark-recapture models incorporating survival and recruitment estimates to inform population viability analyses.
Together, these efforts will refine our understanding of how local ecological conditions shape life-history traits in K. scorpioides and support evidence-based policies for species conservation, particularly in the face of habitat degradation and exploitation. Our findings serve as a foundation for future ecological and demographic research essential for the long-term sustainability of this regionally important freshwater turtle.
We gratefully acknowledge the financial support provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA). We also extend our sincere thanks to the students, researchers, and volunteers who contributed to the long-term data collection efforts. We are particularly grateful to F. Martins, A. Castro, and other colleagues for their valuable feedback during manuscript preparation. Special thanks are due to the residents of Curupu Island for their support and to our dedicated field assistant, Joab.
This study was conducted under permit #14078 issued by ICMBio and was approved by the Animal Ethics Committee of the Federal University of Maranhão (UFMA), under protocol no. 005 374/2010-0. All procedures involving animals complied with relevant ethical guidelines and legal regulations for animal care and collection.
Ethical compliance
All fieldwork involving Kinosternon scorpioides was conducted in accordance with Brazilian environmental regulations and approved by the appropriate institutional and governmental bodies. Research activities were authorized under licenses issued by the Sistema de Autorização e Informação em Biodiversidade (SISBIO).
Handling protocols followed best practices for minimizing stress and injury in wild reptiles. Turtles were manually captured or collected from baited traps and handled for the shortest duration possible. All biometric measurements were taken using non-invasive methods, and individuals were released at their point of capture immediately after processing. During handling, animals were kept moist and shaded to prevent overheating, especially during the dry season.
To ensure individual identification without long-term harm, turtles were marked using a standardized notching system on the marginal scutes of the carapace, following the method of Cagle [33]. This technique is widely accepted and has not been associated with long-term negative effects when performed correctly. All field personnel received prior training in marking, handling, and data collection protocols to ensure animal welfare and data consistency.
Trap-related injuries were rare throughout the study period. Trap design was periodically reviewed and modified to minimize risks, such as by adjusting mesh size and monitoring intervals during peak activity periods.
Capture intensity varied seasonally, with higher recapture success during the rainy season (December–May), when turtle activity increased due to improved water availability. During the dry season (June–November), capture effort was reduced to minimize disturbance during estivation periods. This seasonal adjustment not only improved detection probability but also reduced physiological stress on individuals during times of limited resource availability.
All procedures adhered to the ethical standards established by the American Society of Ichthyologists and Herpetologists (ASIH), the Herpetologists’ League (HL), and the Society for the Study of Amphibians and Reptiles (SSAR), as well as national and institutional animal care guidelines.
- Begon M, Townsend CR, Harper JL. Ecology of Individuals and Ecosystems. Porto Alegre, Brazil; 2007. Available from: https://archive.org/details/begon-2007.-ecologia-de-individuos-a-ecossistemas.-4.-ed
- Stearns SC. The evolution of life history traits: A critique of the theory and a review of the data. Annu Rev Ecol Syst. 1977;8:145–71.
- Congdon JD, Gibbons JW. The evolution of turtle life histories. In: Gibbons JW, editor. Life history and ecology of the slider turtle. Washington (DC): Smithsonian Institution Press. 1990;45–54.
- Silva-da-Silva J, Silva RSR, Lima FCS, Vogt RC. Sexual dimorphism in Kinosternon scorpioides from Marajó Island, Brazil. Rev Biol Trop. 2021;69(2):601–14. Available from: https://doi.org/10.15517/rbt.v69i2.42834
- Iverson JB, Berry JF. Kinosternon scorpioides (Linnaeus, 1766) – Scorpion Mud Turtle. In: Rhodin AGJ, et al., editors. Conservation biology of freshwater turtles and tortoises: A compilation project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. Chelonian Res Monogr. 2011;5:063.1–13. Available from: https://doi.org/10.3854/crm.5.063.scorpioides.v1.2011
- Pritchard PCH, Trebbau P. The turtles of Venezuela. Athens (OH): Society for the Study of Amphibians and Reptiles; 1984.
- Andrade EB. First documented record of Kinosternon scorpioides (Chelonia: Kinosternidae) in the state of Piauí, Northeastern Brazil. Pesqui Ens Cienc Exat Nat. 2019;3(2):216–23. Available from: https://doi.org/10.29215/pecen.v3i2.1288
- Pereira LA, Sousa AL, Cutrim MVJ, Moreira EG. Ecological characteristics of the habitat of Kinosternon scorpioides scorpioides Linnaeus, 1766 (Reptilia, Chelonia, Kinosternidae) in the municipality of São Bento – Baixada Maranhense (Maranhão, Brazil). Bol Lab Hidrobiol. 2007;20(1):9–14.
- Barreto L, Lima LCP, Barbosa SG. Observations on the ecology of Trachemys adiutrix and Kinosternon scorpioides on Curupu Island, Brazil. Herpetol Rev. 2009;40(3):283–6. Available from: https://www.researchgate.net/publication/287527291_Observations_on_the_ecology_of_Trachemys_adiutrix_and_Kinosternon_scorpioides_on_Curupu_Island_Brazil
- Buskirk JR. Natural history notes: Kinosternon scorpioides (Scorpion Mud Turtle). Behavior. Herpetol Rev. 2007;38(3):332.
- Costa FB, Alves FR, Costa AP, Barros ACE, Guerra PC, Sousa AL, Oliveira AS. Ultrasonographic and radiographic determination of egg development of jurarás (Kinosternon scorpioides) in captivity. Pesqui Vet Bras. 2009;29(10):841–6. Available from: https://doi.org/10.1590/S0100-736X2009001000011
- Carvalho‑Jr EAR, Carvalho‑Neto CS, Paschoalini EL. Diet of Kinosternon scorpioides in Serra dos Carajás, eastern Amazonia. Herpetol Rev. 2010;39(3):283–5. Available from: https://www.researchgate.net/publication/260545579_Diet_of_Kinosternon_scorpioides_in_Serra_dos_Carajas_Eastern_Amazonia
- Freiberg M. Turtles of South America. Neptune (NJ): T.F.H. Publications. 1982.
- Lovich JE, Gibbons JW. Age at maturity influences adult sex ratio in the turtle Malaclemys terrapin. Am Nat. 1990;135(1):103–16. Available from: https://doi.org/10.2307/3545132
- Ernst CH, Lovich JE. Turtles of the United States and Canada. Baltimore (MD): Johns Hopkins University Press; 2009.
- Congdon JD, Van Loben Sels RC. Growth and body size in Blanding's turtles (Emydoidea blandingii): Relationships to reproduction. Can J Zool. 1991;69(1):239–45. Available from: https://doi.org/10.1139/z91-038
- Close LM, Seigel RA. Body size and reproductive variation in populations of the turtle Kinosternon subrubrum (Kinosternidae). Herpetologica. 1997;53(3):269–80.
- Andrews RM. Patterns of growth in reptiles. In: Gans C, Pough FH, editors. Biology of the Reptilia. New York: Academic Press. 1982;13:273–320. Available from: https://www.journals.uchicago.edu/doi/abs/10.1086/285385?journalCode=an
- Brown GP, Bishop CA, Brooks RJ. Growth rate, reproductive output, and temperature selection of snapping turtles in habitats of different productivity. J Herpetol. 1994;28(4):405–10. Available from: https://www.jstor.org/stable/1564950
- Litzgus JD, Brooks RJ. Growth in a cold environment: body size and sexual maturity in a northern population of spotted turtles, Clemmys guttata. Can J Zool. 1998;76(5):773–82. Available from: https://doi.org/10.1139/z98-002
- Roosenburg WM, Kelley KC. The effect of egg size and incubation temperature on growth in the diamondback terrapin (Malaclemys terrapin). J Herpetol. 1996;30(2):198–204. Available from: https://www.jstor.org/stable/1565510
- Parmenter CJ. Factors affecting the growth of hatchling turtles. Copeia. 1980;1980(2):299–307.
- Avery HW, Spotila JR, Congdon JD, Fischer RU, Standora EA, Avery SB. Roles of diet protein and temperature in the growth and nutritional energetics of juvenile slider turtles, Trachemys scripta. Physiol Zool. 1993;66(5):902–25. Available from: https://www.jstor.org/stable/30163746
- Germano DJ. Growth and maturity of North American tortoises in response to regional climates. Can J Zool. 1994;72(6):918–31. Available from: https://www.csub.edu/~dgermano/NATortGrowth.pdf
- Turner FB, Medica PA, Bury RB. Growth and survival of the desert tortoise (Gopherus agassizii) in the Mojave Desert, California. Herpetologica. 1987;43(2):151–62.
- Clark DR, Gibbons JW. Growth rates and age at maturity of the turtle Pseudemys scripta elegans. Copeia. 1969;1969(2):253–60.
- Chen TH, Lue KY. Growth patterns of the yellow-margined box turtle (Cuora flavomarginata) in northern Taiwan. J Herpetol. 2002;36(2):201–8. Available from: https://doi.org/10.1670/0022-1511(2002)036[0201:GPOTYM]2.0.CO;2
- Spencer RJ. Growth patterns of two widely distributed freshwater turtles and a comparison of common methods used to estimate age. Aust J Zool. 2002;50(5):477–90. Available from: https://www.publish.csiro.au/zo/zo01066
- Mahmoud IY. Comparative ecology of the Kinosternid turtles of Oklahoma. Southwest Nat. 1969;14(1):31–46.
- Wilbur HM, Morin PJ. Life history evolution in turtles. In: Gans C, Huey RB, editors. Biology of the Reptilia. Vol. 16. New York: Alan R. Liss. 1988;387–440.
- Marengo JA, Alves LM. Climate change in the semi-arid region of Brazil. Reg Environ Change. 2015;15(7):1151–62.
- Silva AL, Silva CA, Costa LC. Climatic characterization of the Amazon coastal region of Maranhão State, Brazil. Braz J Meteorol. 2010;25(3):350–63. Available from: https://doi.org/10.1590/S0102-77862010000300007
- Cagle FR. A system of marking turtles for future identification. Copeia. 1939;1939(3):170–3. Available from: https://www.jstor.org/stable/1436818
- Sexton OJ, Congdon JD. A simple and reliable system for marking hard-shelled turtles: The North American Code. Herpetol Rev. 1974;5(3):97–8.
- Gibbons JW, Lovich JE. Sexual dimorphism and the evolution of body size in turtles. Herpetologica. 1990;46(2):129–39.
- Ernst CH, Lovich JE, Barbour RW. Turtles of the United States and Canada. Washington (DC): Smithsonian Institution Press; 1994. Available from: https://pubs.usgs.gov/publication/81501
- Moll D, Moll EO. The ecology, exploitation, and conservation of river turtles. Oxford: Oxford University Press. 2004.
- Vazzoler AEA de M. Reproductive biology of teleost fish: theory and practice. Maringá: EDUEM; 1996. Available from: https://www.scienceopen.com/document?vid=f698c980-022c-4669-b1fb-be792ddcdcaa
- Christmann EA. Basic concepts of statistics for biology. Porto Alegre: Editora Globo; 1978.
- Magnusson WE, Lima AC, Costa VL, Lima OP. Growth of the turtle Phrynops rufipes in Central Amazonia, Brazil. Chelonian Conserv Biol. 1997;2(4):576–81.
- StatSoft Inc. Statistica (Version 10) [Computer software]. Tulsa (OK): StatSoft Inc.; 2010. Available from: https://www.statsoft.com
- Barreto L, Neckel‑Oliveira S, Ribeiro LES, Garcez RBM, Calvet MCR, Oliveira CC, van Zuidam BG, Roessink I, van Nes EH, Peeters ETHM. Seasonal variation in the population parameters of Kinosternon scorpioides and Trachemys adiutrix, and their association with rainfall in seasonally flooded lakes. Herpetol Conserv Biol. 2020;15(2):457–66. Available from: https://research.wur.nl/en/publications/seasonal-variation-in-the-population-parameters-of-kinosternon-sc
- Medeiros AM, Rodrigues CAL, Pereira EP, Cunha FAG, Ferreira DIS, Chaves EP, Chaves LPFA, Lima BC, Pereira LA, Sousa AL. Reproductive biology and habitat characterization of the scorpion mud turtle Kinosternon scorpioides (Linnaeus, 1766) in the Amazon-Cerrado ecotone region, northeastern Brazil. Braz J Biol. 2024;84:e285306. Available from: https://doi.org/10.1590/1519-6984.285306
- Acuña-Mésen R, Castaing A, Flores F. Ecological aspects of the distribution of terrestrial and semi-aquatic turtles in the Central Valley of Costa Rica. Rev Biol Trop. 1983;31(2):181–92. Available from: https://archivo.revistas.ucr.ac.cr//index.php/rbt/article/view/24932
- Berry JF, Seidel ME, Iverson JB. A new species of mud turtle (Kinosternon) from Jalisco and Colima, with notes on its natural history. Chelonian Conserv Biol. 1997;2(3):329–37.
- Mora JM, Castañeda FE. Terrestrial movements, activity patterns and habitat use by Kinosternon scorpioides (Testudines: Kinosternidae) in Palo Verde National Park, Costa Rica. Phyllomedusa. 2022;21(1):3–15. Available from: https://revistas.usp.br/phyllo/article/view/199314
- Ferreira PFG, Silva Filho E, Marques LC, Leal RP, Marques JRF. Scorpion mud turtle breeding in the Amazon: zootechnical and environmental activity for the species conservation. Herpetol J. 2024;34(4):256–65. Available from: https://doi.org/10.33256/34.4.256265
- Freitas RS, Rocha KS, Monteiro LH, Alexandre TF, Monteiro TRM, Honorio BET, et al. Detection of Pathogenic Leptospira in Captive Chelonians (Kinosternon scorpioides—Linnaeus, 1766) in the Brazilian Amazon. Animals. 2024;14(9):1334. Available from: https://doi.org/10.3390/ani14091334
- Pearl R. The rate of living. Ann Arbor (MI): University of Michigan Press. 1928.
- Wilbur HM. A growth model for the turtle Chrysemys picta. Copeia. 1975;1975(2):337–43. Available from: https://www.jstor.org/stable/1442888
- Dunham AE, Gibbons JW. Growth. In: Gibbons JW, editor. Life history and ecology of the slider turtle. Washington (DC): Smithsonian Institution Press. 1990;171–82.
- Bury RB. Population ecology of freshwater turtles. In: Harless M, Morlock H, editors. Turtles: Perspectives and research. Malabar (FL): Krieger Publishing Company. 1989;417–34.
- Martins FI, Franco LS. Estimates of growth of the Atlantic Forest freshwater turtle Hydromedusa maximiliani. J Herpetol. 2008;42(1):54–60. Available from: https://www.jstor.org/stable/40060482
- Medeiros AM, Araújo LSR, Mesquita SL, Aragão NRC, Rodrigues CAL, Chaves EP, et al. Traditional knowledge on the use of turtles in a protected area of the Amazon in Maranhão (Brazil): A conservation proposal. Hum Ecol. 2023;51:567–78. Available from: https://doi.org/10.1177/02780771231176468
- Silva JM, Alves RRN, Oliveira RR. The trade of Kinosternon scorpioides on Marajó Island, Brazilian Amazon: From hunting to consumption. Herpetol J. 2017;27(4):233–9. Available from: https://www.periodicos.capes.gov.br/index.php/acervo/buscador.html?task=detalhes&source=all&id=W2808538783
- Maranhão Institute of Socioeconomic and Cartographic Studies (IMESC). Environmental situation of Maranhão Island. Maranhão (Brazil): IMESC; 2011.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
