Different Approaches to establish soil health and to combat Phytophthora cinnamomi
1Department of Animal Production, Faculty of Veterinary Medicine, Complutense University of Madrid, 28040 Madrid, Spain.
2Soluciones dehesa Sana, S.L., High-tech incubator, 06800 Mérida, Spain
3Campojerez, S.L., 06380 Jerez de los Caballeros, Spain
4Ingulados, S.L., 10001 Cáceres, Spain
5Joselito S.A., Research and Development Department, 37770 Guijuelo, Spain
Author and article information
Cite this as
Beatriz IR, Blanca CR, Angela CS, Luis GNJ, Antonio R, et al. (2024) Different Approaches to establish soil health and to combat Phytophthora cinnamomi. Open J Plant Sci. 2024; 9(1): 006-015. Available from: 10.17352/ojps.000059
Copyright License
© 2024 Beatriz IR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The sustainability of ecosystems is threatened especially in unique spaces that traditionally have a great wealth of biodiversity. Since the beginning of the 80s, the multifactorial syndrome called “la seca” has been the main disease that affects the holm oaks and cork oaks characteristic of the Mediterranean forest, and that gradually dries the trees until they die. This pathology is multicausal and one of the agents with the greatest lethal power is Phythoptora cinamomi, of the Protista genus, considered one of the 100 worst invasive species in the world.
Current solutions are based on preventive cultural practices and the use of palliatives, mainly chemicals. Research is advancing rapidly in the field and biocontrol solutions are being incorporated, such as the use of biological phytosanitary products, such as microorganisms or plant extracts with direct action against the pathogen, but which still present major deficiencies. The global overexploitation that has led to the rise of these pathogenic microorganisms leads to an impoverishment of the natural microbiome of the pastures, so it is necessary to reestablish this microbiome and the balance of the soils so that they recover their suppressive characteristics against P. cinnamomi and other pathogens that could threaten the disease. The objectives of this review are to raise awareness of the current problems in the Mediterranean forest ecosystem, and its relationship with a global phytopathogen such as Phythopthora cinnamomi, and to provide new strategies for soil regeneration.
Global overexploitation has led to a global imbalance of nature and soil (EU Report 33/2018). The increasing number of pathogenic microorganisms results in the degradation of the natural microbiome of the Mediterranean forest called “dehesas” (agroforestry systems of the southwestern Iberian Peninsula where the grassland is combined with species of the genus Quercus). Therefore, it is imperative to restore this microbiome and soil balance to regain their ability to suppress pathogens like Phytophtora cinnamomi (P. cinnamomi) and other pathogens that may threaten them. Ecosystems worldwide are under threat, requiring tailored solutions on a case-by-case basis to restore this lost balance.
Around the world, the Phytophthora family of pathogens is known as the “plant destroyer” due to the fact that it causes significant annual economic losses costing between 2 and 7 billion dollars in agricultural systems, not to mention unquantifiable losses in natural ecosystems [1]. In the specific case of damage in the southwest of the Iberian Peninsula, more than 30,000 hectares are affected by these pathogens, with an annual growth rate of more than 0.5% between 1957 and 2013. In infected areas, the annual tree mortality rate is approximately 15 times higher than in unaffected areas.
Currently, there are 4.5 million hectares of dehesa in the southern and western European regions, with the southwestern Mediterranean regions being the most affected by the death of Quercus trees, with 1.2 million hectares damaged [2]. The dehesa is an ecosystem characterized by the presence of trees from the Quercus genus (primarily holm oaks -Quercus ilex- and cork oaks -Quercus suber-). These ecosystems are crucial sources of biological resources, supporting the development of rural populations and generating wealth through high-value food products, the most notable example being acorn-fed Iberian ham [3].
Many factors, such as global warming, abiotic and biotic pollution, overexploitation of water resources, and poor environmental management, have led to a significant increase in phytosanitary issues affecting holm oaks and cork oaks. These factors resulted in the presence of over 5,000 outbreaks of the so-called “la seca” (Figure 1)) in the southwestern Mediterranean regions, which has resulted in the death of approximately 1,000,000 holm oaks and cork oaks, with a total affected area of 100,000 hectares [4]. This decline in the holm oak population poses a threat to economic activities associated with the dehesa, such as the extensive livestock sector, which produces high-quality products mainly from cattle and Iberian pigs.
Although several studies address this problem of drying out, none of them agree on the factors responsible for it. However, all of them agree on the presence of a phytopathogen called Phytophthora cinnamomi. This microorganism, belonging to the class Oomycetes and to the kingdom Chromista [1], carries out its entire life cycle in soil (asexual and sexual phases), where humidity is a critical factor for its development and propagation [5]. P. cinnamomi (hereinafter, “Pc”) can grow as a saprophyte on dead organic matter or as a parasite, affecting approximately 5,000 species of woody, shrubby, and herbaceous plants [6], including holm oaks and cork oaks. P. cinnamomi typically infects roots, but it can also invade woody stems, particularly through suberised periderms [7]. Its growth in the root system causes root rot and interferes with the absorption of raw sap, resulting in leaf chlorosis, among other effects. Following infection with P. cinnamomi, hosts may die quickly or may be asymptomatic for many years.
In addition to its ability to spread rapidly, P. cinnamomi is highly resistant to conventional eradication treatments. Most fungicides have no effect on Phytophthora species, with the exception of metalaxyl [8] or certain phosphonates [14,15]. Instead of eliminating the pathogen, the application of fungicides can induce various natural resistance mechanisms in the plant [9]. However, it is important to highlight that the use of pesticides can have negative effects on the environment, such as soil and water contamination, so it is advisable to minimize the use of these compounds.
The ecological significance of these agroecosystems underscores the urgency to seek alternative eco-friendly methods of pest and disease control, looking for strategies to reduce the use of synthetic products [10]. It is imperative to find alternative solutions that increase the likelihood of success in eliminating P. cinnamomi, such as biological control. Currently, there is no effective treatment for this disease. To combat this pathology and P. cinnamomi (Pc), integrated management plans are being developed that primarily focus on prevention and change in the management of dehesas, as summarised in the interregional PROdehesa MONTADO project’s management manual [11].
Firstly, genetic improvement strategies for holm oak and cork oak species are being explored. This field is still in the research phase, with no established market for commercial species. There are several species selection and improvement programs, as the intraspecific genetic variability in holm oaks and cork oaks is considerable and can be leveraged to select more tolerant genotypes [12, 13]. While this approach is promising, it is primarily applicable to reforestation efforts and may not fully address the needs of mature trees.
Another approach is chemical control with the use of fungicides, the application of calcium amendments, and/or organic amendments. In this respect, most fungicides are ineffective against Phytophthora spp., although some phenylamides, such as metalaxyl [8], or some phosphonates, such as potassium phosphite and fetal-Al, can be effective [14,15] (Figure 2). In Portugal, the only product officially approved for holm oak and cork oak is fetal-Al [9], and in Spain, it is only approved for agricultural and ornamental plants, but it is also applied to holm oaks and cork oaks. This fungicide has proved to be a good alternative to potassium phosphite, which is not approved in Portugal or Spain. Unfortunately, these products require repeated applications, as their effectiveness diminishes over time [16], and prolonged use of certain fungicides, such as metalaxyl, can lead to the development of resistance and Pc mutations associated with these compounds [17,18].
Additionally, certain calcium compounds such as CaO, CaCO3, or CaSO4, have been shown to reduce the severity of this disease in mustard plants [19]. Unfortunately, the calcification of soil does not impact the viability of resistant pathogen spores (chlamydospores), which remain a source of inoculum and infection.
As regards the availability of phytosanitary products on the market, there are only four products approved in Spain for use on holm oaks and cork oaks (Table 1).
As can be seen in Table 1, all of them are chemical in nature and the main use of the only biological product, which was only initially registered in the Netherlands (and therefore we cannot ascertain its origin), is as a treatment against Lepidoptera on tomatoes and greenhouse crops [20]. In order to overcome the problems posed by the use of chemical pesticides, biological control (or biocontrol) strategies are also being researched and developed, as described in detail below.
This manuscript presents the beginning of a research project that we have called DEHELIFE (CDTI-ABS 20230188 – Ministry of Science, Innovation and Universities) with the objective of developing soil bioregulatory products based on native microorganisms – such as T. hamatum, T. koningii, T. koningipsis and T. atroviridae – and their characterized biocomposites.
As stated in such a complete objective, the innovation of the project is based on the approach of the bioregulatory product (inhibitor and bio stimulator) and not just a pesticide, as well as its nature: indigenous live microorganisms and active compounds characterized as the result of controlled fermentation. This guarantees immediate action of the product and at the same time, sustainable over time. However, this project not only aims to focus on microorganisms but also on plants through research into indicators that show the state of the immune system of holm oaks and cork oaks, as well as research into the metabolism linked to stress. Extensive scientific knowledge will be obtained to provide resources to combat drought and soil regeneration, as well as new products that help stop the decline of such a valuable ecosystem on which many human families, animals, and plants depend.
The main objective of this review is to raise awareness of the current problems in the Mediterranean forest ecosystem, and its relationship with a global phytopathogen such as Phythopthora cinnamomi, and to provide new strategies for soil regeneration.
Biocontrol strategies with plant species
Research conducted by Serrano [21] has demonstrated the sensitivity of several species to Pc, particularly Lupinus luteus (lupin). Not only is it much more susceptible to infection, but it can also act as a source of inoculum, facilitating infection of the roots of other plants.
By contrast, there are also plant species resistant to the microorganism, such as ash, tree heather, oat, or flax-leaved daphne, among others [22]. As the cultivation of susceptible leguminous species such as lupin facilitates the spread of Pc, the presence of these resistant species, such as those described in Table 2, could prevent or significantly reduce the growth of the pathogen.
Research suggests that this type of plant could synthesize compounds with an antifungal activity which, in addition to protecting the plant itself, would help the regeneration of infected land and help the defense of holm oaks and cork oaks in these areas [23].
The most interesting studies on the beneficial activity of some of these plant species have focused on the matagallo plant (Phlomis purpurea), present in the southwest of the Iberian Peninsula, and its ability to produce bioactive compounds with antifungal potential [23]. It has been observed that this species has the ability to inhibit the growth of plant pathogens, and more specifically, its roots produce compounds capable of protecting from infection by Pc in vitro.
However, it has been observed that in plant extracts, such as Phlomis purpurea, the bioactive component is found in minute concentrations [24]. Its scarcity, the fact that it is always found in the plant as part of complex mixtures extracted from its tissues, and the fact that, due to its complex structure, it cannot be chemically synthesized, make its purification and development for commercial use as a phytoprotective agent a difficult objective, despite its great potential to protect these forest ecosystems, which are particularly important for the economy of Extremadura.
Furthermore, these challenges are not the only obstacles encountered in the development of biocontrol solutions for Pc.
While Cardillo y Acevedo [22] documented data on resistant species presented in Table 2, an earlier publication by Álvarez, et al. [25] observed root rot in lavender and rosemary, species included in that list (Characterization of phytophthora nicotianae isolates causing collar and root rot of lavender and rosemary in SPAIN., 2007). This demonstrates that there is uncertainty about which species are truly resistant to Pc, as this pathogen continues to mutate and adapt to its environment, infecting more plant species over time. For example, wild olive (Olea oleaster) is included in the list but was later found susceptible to infection by another Phytophthora species in 2019 [26].
In light of the above, the following can be considered the main issues with using plant species for biocontrol:
- Despite the effectiveness of preventive buffer strips and the suppressive plant cover, not all species are compatible with agro-livestock activities.
- There are technical and economic feasibility issues related to developing products based on extracts or isolated bio-compounds.
- P. cinnamomi exhibits a rapid generation of resistance, resulting in fewer and fewer plant species resistant to this pathogen.
Microorganisms as biocontrol of P. cinnamomi
Suppressive soil isn’t solely understood as the coexistence of plants and Pc. Within the soil of any ecosystem, a diverse array of microorganisms, including fungi, are adapted and collectively form the microbiota, performing fundamental functions for the ecosystem. Fungi are mainly known for their saprophytic properties and are decomposers of dead matter. They inhabit the final stages of the decomposition process and thus allow molecules, which once formed part of living organisms, to pass into the soil so that they can be used by plants growing in the soil. From an evolutionary perspective, starting from this fundamental saprophytic function, they gradually adapt to the environment, diversifying their functions, specializing in different areas that derive from the relationships they form with different organisms coexisting in the ecosystem. Two of them are particularly noteworthy for their potential uses in the fight against plant pathogens: mycoparasitism and plant immunity promoters.
Some fungal species changed from saprophytes to mycoparasites, i.e. they changed their source of nutrients from obtaining nutrients from dead matter to feeding on other fungi in the environment. This property can be used to deal with plant pathogenic fungi affecting plant health, i.e. to use fungi for their mycoparasitic properties as biocontrol agents. There are two main mechanisms by which these mycoparasitic fungi possess antimicrobial activity, by physical competition and by production of metabolites with antimicrobial activity. The mycoparasite fungus is able to recognize its prey, spread its hyphae around it, and produce lytic enzymes to degrade cellular components. These same lytic proteins are molecules with antimicrobial activity, but they also produce secondary metabolites with antifungal activity. Some of these proteins, such as cellulases, proteases, and chitinases, are widely used in industries such as paper and textiles to treat raw materials. In addition, they produce small secondary metabolites such as triterpenes or peptabiols that exhibit a multitude of biological activities, including antimicrobial activity, among others.
For example, the use of other organisms to control the presence and infection of P. cinnamomi has been tested and the results are promising, although further studies are needed [27]. Bosso et al., [28]. showed that Byssochlamys nivea and Scopulariopsis brumptii in laboratory studies were able to inhibit the growth of P. cinnamomi and P. cambivora and reduce the mortality of chestnut plants [28]. Supporting the use of biological control, Méndez-Bravo et al., [29]. reported that two rhizobacteria, closely related to Bacillus acidiceler, were able to inhibit the growth of P. cinnamomic in vitro by 76%, suggesting that these bacteria could be used for biological control of oomycetes [29]. Finally, Trzewik et al., [30]. reported a practical likelihood of biological protection against P. cinnamomic or Piriformospora indica, an endomycorrhizal-like fungus, on two cultivars of rhododendron plants [30]. Bacterial genera commonly used in agriculture include Azospirillum, Rhizobium, Pseudomonas, and Bacillus.
With regard to mycorrhizal fungi, Trichoderma spp. is one of the most studied filamentous fungi. Trichoderma (teleomorph Hypocrea) is a genus of filamentous fungi that feed on other fungi (mycotrophism) and is a ubiquitous colonizer in almost every environment. Today, there are more than 400 recognized species [31]. The genus includes mainly non-pathogenic soil fungi, considered opportunistic avirulent plant symbionts, and root colonizers, which, in several cases, produce compounds that stimulate plant protection and growth.
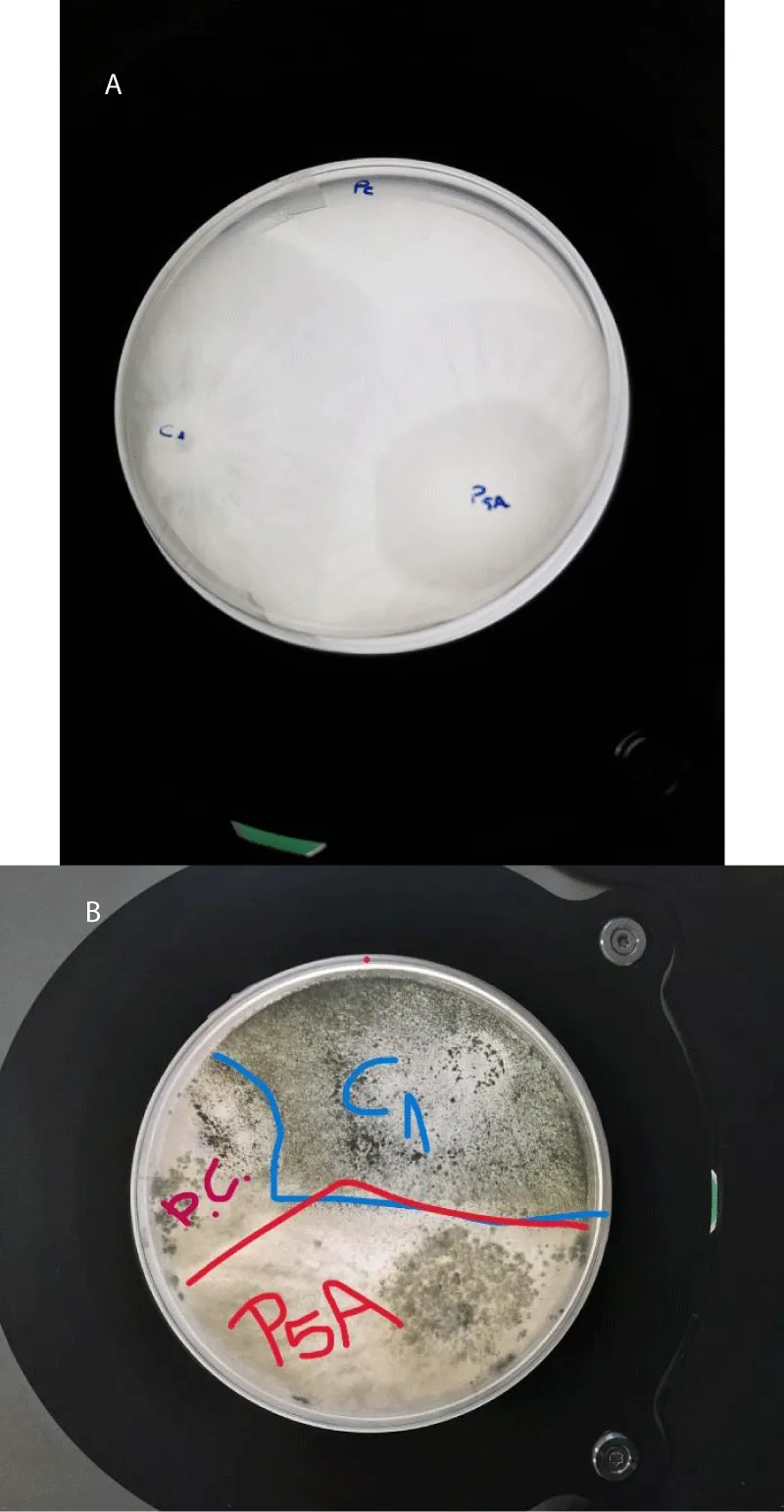
Some Trichoderma species can control inoculum levels through inhibition or even mycoparasitism [32]. Some literature reviews suggest that especially Trichoderma harzianum, T. virens, and T. asperellum, are very promising fungal species in the control of different Phytophthora species [34], as studied in multiple crops such as pepper, chilli, avocado, and Cornaceae. Other studies have shown similar results for biological agents controlling several soil-borne plant pathogenic fungi [10-33] including P. cinnamomi [34]. Our preliminary data in the laboratory working with T. hamatum, T. koningii, T. koningipsis, and T. atroviridae indicate positive results as you can observe in Figure 3. C1 and P5A are products with different mixed strains of these Trichoderma (Figure 3).
There are currently approved products available on the market that are based on Trichodermas and other microorganisms, such as bacillus but all of them are studied and recommended for use on horticultural crops and other important species such as avocado, but very few studies focus on the specific issues faced by the dehesa. On these issues, studies by the University of Córdoba have been noteworthy. Microbiome populations have been determined through metabarcoding studies of genetic material in several areas of dehesa in Andalusia [35]. On the basis of this study, further research has been carried out on the selection of mycoparasitic Trichoderma families as P.cinamomi inhibitors.
Complexes with T. viridae and T. harzianum have been tested on Phytophthora cinnamomi-infected holm oak seedlings in two contrasting holm oak ecotypes, one considered highly susceptible and the other considered tolerant to the pathogen in the greenhouse, and seedlings were monitored for survival analysis and review of morphological and physiological attributes [32].
This research group’s most recent studies detail a very interesting and thorough study on the mycoparasitic ability of the genus Trichoderma on P. cinamomi but do not elaborate on the molecules involved in them, neither hydrolytic proteins nor small secondary metabolites. No metabolites with antimicrobial and immunomodulatory activity produced by Trichoderma have been identified.
In addition, among the results obtained from the characterization of the soil microbiome by metabarcoding, significant variability in the microbiome populations between dehesas has been observed [35], highlighting the need to investigate specific solutions for each ecosystem. This fact justifies the need to investigate organisms and bioactive components native to our ecosystem to combat P. cinnamomi in the dehesa of Extremadura.
Biological control against P. cinnamomi
As mentioned above, as a technology that counteracts classical chemical control, there is a need to utilize biocontrol strategies, primarily based on the use of pathogen-suppressive microorganisms.
This approach is highly applied in other larger sectors of the global economy, such as horticulture or intensive forest management. There are also some specific solutions against pathogens of the Phytophthora family, such as P. nicotianae in tobacco or P. capsici in pepper. These solutions are marketed by companies such as the GOWAN Group (Blindar® for vineyards and horticulture, Remedier® for horticulture), Certis (VALCURE® for horticulture and fruit trees), the Spanish spinoff Biocontrol Technologies SL, Bioworks (only in the USA and Canada) and Agrogenia Biotech.
Within the families of microorganisms to be used, there are already commercial products with Bacillus and Trichodermas but, similarly, they are only focused on crops for human consumption. The only compound registered in Spain for holm oak and cork oak is Probelte’s BELTHIRUL 16 SC, composed of Bacillus thuringiensis kurstaki.
As explained above, these compounds could be intended to be used in the dehesa, but, firstly, they have not been specifically developed against P. cinnamomi in holm oaks and cork oaks; and secondly, they are not organisms of the indigenous microbiome, which could lead to an imbalance of the ecosystem.
In addition, Rodríguez et al. [13] and Rodríguez y Rodríguez [2] have published the most technologically advanced information. Since 2019, these authors have studied the microbiome of the dehesa, have identified mycoparasitic inhibitor microorganisms of Pc, and are evaluating compounds of specific microorganisms with T. viride and T. harzianum.
Biostimulants
Biostimulants are substances or microorganisms that promote and/or enhance plant growth, development, metabolism, and/or tolerance to abiotic stress without being fertilizers or pesticides [37]. As agreed by scientists, regulators, and other stakeholders, several main categories of widely recognized biostimulants have been identified, namely:
o Chitosan and other biopolymers
o Hydrolysates of proteins and other nitrogenous molecules
o Humic substances
o Extracts from marine algae and botanicals
o Beneficial bacteria
o Beneficial fungi
o Inorganic compounds (i.e. Al, Co, Na, Se, Si)
Globally, these solutions are attracting market interest and R&D efforts as a necessary alternative to the problems of the agricultural sector. Biostimulants shift the focus from direct combat against pathogens to a soil and ecosystem recovery approach.
As regards biostimulant microorganisms [38], this group includes mainly bacteria, yeasts, and filamentous fungi. They are isolated from soil, plants, and other organic materials. They are applied to soil or seeds and can directly or indirectly contribute to enhancing crop productivity (2). Microorganisms can directly affect crops by establishing mutual symbiotic associations (e.g., mycorrhizae), or indirectly by enhancing the bioavailability of nutrients to plants (2). According to the latest European fertilization regulation [39] the microorganisms Azotobacter spp., Mycorrhiza, Rhizobium spp., and Azospirillum spp. are recognized as biostimulants. This field holds significant potential but is still in the developmental stage, and with few microbiological solutions.
There are no documented instances of biostimulant use in holm oaks or cork oaks, and at the academic level, only a handful of studies have explored the application of biostimulants derived from Ascophyllum nodosum on oak trees [40].
Diagnosing dried-out plants affected by “la seca”
As already mentioned, diagnosing P. cinnamomi is crucial for implementing treatment and preventive strategies to avoid its spread. However, existing guidelines and practices rely solely on symptom detection to guide sample collection and laboratory confirmation of the infection.
According to the CICYTEX dehesa Montado Observatory, the presence of P. cinnamomi can be identified by observing the following characteristics:
The tops appear less bushy than usual or have lost more leaves than usual (Figure 4), and the uppermost branches appear leafless (dotted). It is also very common to find trees that die quickly during the summer with all their leaves turning dry and brown (sudden death).
Affected trees are often found in watercourses or areas where water accumulates, particularly during rainy periods. Sometimes, the presence of impermeable soil layers may contribute to deep waterlogging, which may not be readily observable on the surface.
To confirm the presence of Phytophthora, it is necessary to collect soil and root samples on which molecular techniques, such as ELISA or PCR assays, will be performed. It is important to note that insufficient or improper sampling may result in a false negative result.
In addition, patterns are recognized, and models have been developed to predict their dispersion [41, 42]. For example, it is known that outbreaks typically advance downhill in the direction of water flow, leaving dead trees and the most damaged specimens uphill. Often, outbreaks can expand in a more or less circular manner. Dispersal studies have also been carried out and orthophoto detection methods are being sought, but these are all based on visual observation of tree phenotypes.
Stress and immunology in holm oaks and cork oaks
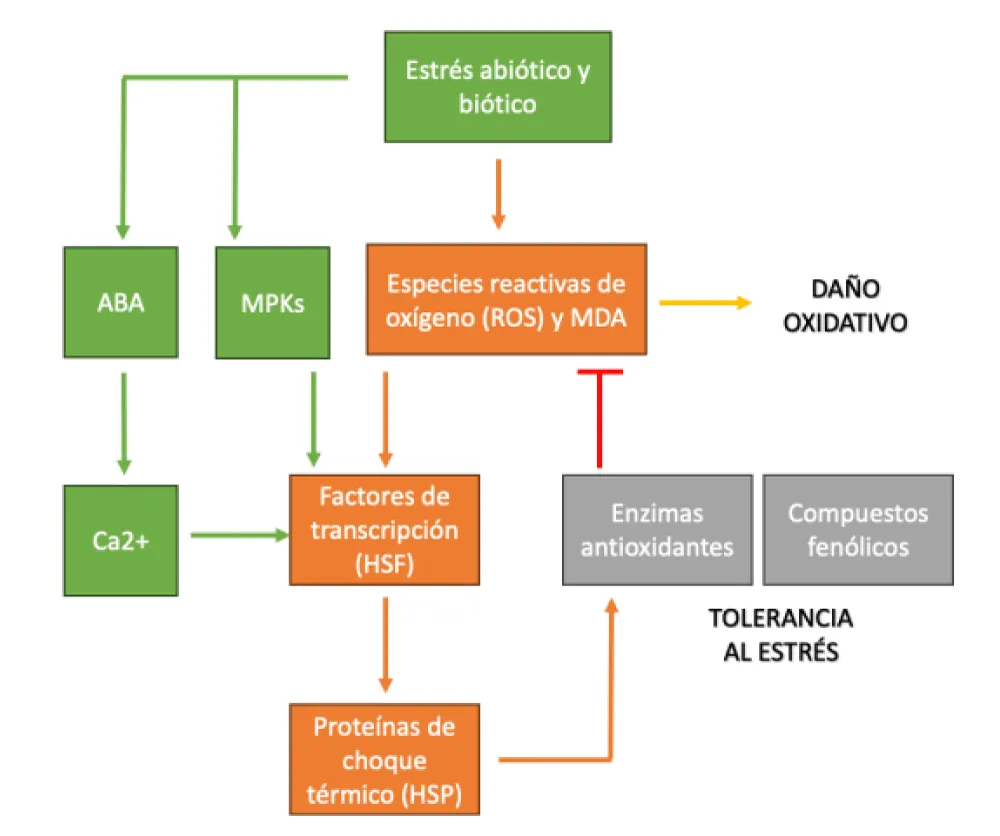
Phenotypic or symptomatic observation is the most widespread traditional method to assess stress levels of plant species, including holm oaks and cork oaks. Furthermore, current knowledge of molecular indicators of stress and plant immunology can provide tools for assessing the condition of trees in dehesas. In Figure 5 we can show up the signaling pathways in response to stress in plants.
Unlike animals, plants lack specialized immune cells and an adaptive immune system. However, they possess an innate immune system that allows them to interact with beneficial microbes or defend themselves against pathogens. This system relies on innate immune receptors that recognize invasion signals. There are two types of immune responses: pattern-triggered immunity (PTI) and effector-triggered immunity (ETI).
Activation of Pattern Recognition Receptors (PRRs) leads to cellular calcium signaling and the activation of protein kinases, resulting in transcriptional reprogramming that promotes defense. Recognition of pathogenic effectors by nucleotide-rich leucine repeat (NLR) receptors involves immune regulators and salicylic acid (SA) signaling.
Plants respond in a variety of ways to biotic and abiotic stresses, ranging from changes in gene expression and physiology to adjustments in plant architecture and metabolism. The response is influenced by factors such as the cause, duration, and intensity of the stress, the genotype of the plant, the type of cell exposed, and the stage of development [43].
Several mechanisms and compounds have been identified in current research as components of a plant’s defense mechanisms and can be used to determine its stress levels. Specifically, phenolic compounds, lipid peroxidation markers, and antioxidant enzymes are noteworthy.
Phenolic compounds
In response to biotic stress, plants activate their defense mechanisms leading to the induction of a broad spectrum of antimicrobial compounds, some of which may be species-specific. Induced resistance is regulated by a network of interconnected signal transduction pathways in which phenolic compounds are vital signaling molecules [44].
Previous studies on various species have shown that stress induced by different abiotic and biotic factors can modify the composition of phenolic compounds such as flavonoids and tannins in different anatomical parts of plants [45]. These secondary metabolites exhibit high antimicrobial activity, are strong inhibitors of digestive proteases, and accumulate mainly in the leaves, stems, and roots of trees [46,47].
With regard to trees of the genus Quercus, some authors have described the content of phenolic compounds under different abiotic conditions, such as cold and drought [48]. Regarding the response to P. cinnamomi, studies have been conducted on the variations of these compounds in chestnut and their dependence on the temperature of the environment [45].
Despite the existence of published studies on the effects of biotic stress on the chemical defenses of holm oaks and cork oaks [49;50], few studies have focused on the combined effect of abiotic and biotic stress factors on the chemical defenses of these tree species and their geographic variability [12].
Malondialdehyde (MDA)
The measurement of malondialdehyde (MDA) content has long been used as a marker for lipid peroxidation in studies related to oxidative stress in plants exposed to abiotic and biotic factors [51]. Increased levels of reactive oxygen species (ROS) have been observed in Quercus spp. in response to abiotic (e.g. drought) and biotic stress (e.g. charcoal disease caused by the fungi Biscogniauxia mediterranea and Obolarina persica), with the intensity of this response being heightened when both stresses are combined [52,53].
Associated markers (MDA increase) have been identified in potato leaves in response to infection by Phytophthora infestans, the causal agent of late blight, and could serve as a marker of abiotic stress in the genus Quercus. In experiments conducted by Morcillo et al.,[54]., higher MDA concentrations were detected in holm oak somatic embryos treated with filtered extracts of Phytophthora cinnamomi oomycetes, although MDA contents were not significantly different from those of controls conducted [54].
Antioxidant enzymes
The metabolism of reactive oxygen species (ROS) is another major plant response to stress. Under stressful conditions, the dynamic equilibrium is broken, and excessive ROS accumulation, oxidative stress, and plant death occur in severe cases [55]. To protect themselves from oxidative stress, plants produce antioxidant enzymes and other non-enzymatic substances that neutralize ROS [56]. Antioxidant enzymes include peroxidase (POD), superoxide dismutase (SOD), ascorbate oxidase (APX), and catalase (CAT) [57].
In recent research by Xiong et al., [58], an increase in the activity of the antioxidant enzymes POD, SOD, and CAT was observed in four oak species subjected to drought stress [58]. In addition, Mohammadi et al.,[59] described an increase of SOD, POD, and CAT activity and non-enzymatic substances such as phenolic compounds in potato leaves infected by Phytophthora infestans, the causal agent of late blight Mohammadi et al., [59]. There are no known studies on holm oaks or cork oaks.
Transcriptomics
The transcriptome is the total set of RNA transcripts present in any cell or biological system under specific physiological and environmental conditions. They are used to identify genes involved in plant deterioration/decay and to decipher the relationship between this phenomenon and biotic and abiotic stress.
In their study, Guerrero-Sanchez et al., [59] identified 12 candidate reference genes that were not differentially expressed in Quercus ilex seedlings under drought stress conditions [59]. These include actin, GAPDH, and β-tubulin, which are considered to be the most stable and reliable candidate reference genes (data not published). On the other hand, infection of cork oak trees with Phytophthora cinnamomi revealed increased levels of proteins associated with the assembly of protein-DNA complexes, lipid oxidation, endoplasmic reticulum stress response, and metabolic processing of pyridine-containing compounds in leaves [61]. The most significant variations were observed in heat shock proteins (Hsp90-1), among others.
Conclusion
In conclusion, while academic knowledge exists regarding mechanisms against phytopathogenic agents, it is necessary to develop strategies to prevent or mitigate mediterranean forest deterioration.
The current technical needs urgently required in combating drought can be summarised as follows:
The a need to find effective alternatives to chemical pesticides that have not been successful in slowing down the progression of the disease.
To find strategies that overcome the limitations associated with using isolated bio compounds against P. cinnamomi, which include technical and economic feasibility issues, as they are mainly extracted from plant extracts. Moreover, these are treatments that need to be repeated over time, as the active ingredients have a certain shelf life.
With regard to biocontrol approaches based on microorganisms, indigenous microorganisms are required, not only as inhibitors but also as biostimulators and soil health restorers through the microbiome. In addition, a mixed approach must be taken, in which not only the microorganism is known and provided, but also the biocomponents involved are known and their application to soils is monitored.
Not only to combat P. cinnamomi but to restore the health of the soil, thereby restoring health to the entire microbiome and ecosystem, by creating suppressive environments in which the pathogen cannot thrive.
- Hardham AR, Blackman LM. Phytophthora cinnamomi . Mol Plant Pathol. 2018 Feb;19(2):260-285. doi: 10.1111/mpp.12568. Epub 2017 Aug 22. PMID: 28519717; PMCID: PMC6637996.
- Rodríguez EL, Rodríguez AB. La dehesa en Extremadura: caracterización y dinámicas a partir del Sistema de Información de Ocupación del Suelo de España (SIOSE) y comparativa con SIGPAC. Cuadernos Geográficos De La Universidad De Granada [Internet]. 2019 Dec 18;58(3). Available from: https://doi.org/10.30827/cuadgeo.v58i3.8641
- Sayadi S, Parra-Lopez C, Caño-Bergara B, Garcia-Moreno A. Valor económico, social y ambiental de la dehesa . Un análisis multifuncional desde la perspectiva de la sociedad andaluza. Informe final del Proyecto LIFE bio dehesa LIFE11 BIO/ES/000726. España. Ecosistema de dehesa : Desarrollo de políticas y herramientas para la gestión y conservación de la biodiversidad; 2019. Anexo D.1.2. Avaible from https://www.uco.es/investigacion/proyectos/bio dehesa /wp-content/uploads/Valor-econ%C3%B3mico-social-y-ambiental-de-la- dehesa .pdf
- Neurona. Cicytex Instituto del corcho, la madera y el carbon vegental [Internet].Neurona; 7 May [citada el 22 de marzo del 2024].Disponible en http://neurona.gobex.es/centros/instituto-del-corcho/
- Zentmyer GA. The effect of temperature on growth and pathogenesis of phytophthota cinnamamo and on growth of its avocado host. Phytopathology {Internet}.26 Jan 1981;71(9). Available from :925-928. 0031-949X/81/09092504/$03.00/0
- Jung TS, Chang TT, Bakonyi J, Seress D, Pérez‐Sierra A, Yang X, et al. Diversity of Phytophthora species in natural ecosystems of Taiwan and association with disease symptoms. Plant Pathology [Internet]. 2016 Jul 11;66(2):194–211. Available from: https://doi.org/10.1111/ppa.12564
- O’Gara E, Howard K, McComb JA, Colquhoun IJ, St J Hardy GE. Penetration of suberized periderm of a woody host by Phytophthora cinnamomi. Plant Pathology [Internet]. 2014 Jun 5;64(1):207–15. Available from: https://doi.org/10.1111/ppa.12244
- González M, Caetano P, Sánchez ME. Testing systemic fungicides for control of Phytophthora oak root disease. Forest Pathology [Internet]. 2017 Mar 13;47(4). Available from: https://doi.org/10.1111/efp.12343
- Romero MÁ, González M, Serrano MS, Sánchez MEV. Trunk injection of fosetyl‐aluminium controls the root disease caused by Phytophthora cinnamomi on Quercus ilex woodlands. Annals of Applied Biology [Internet]. 2019 Mar 22;174(3):313–8. Available from: https://doi.org/10.1111/aab.12503
- Thambugala KM, Daranagama DA, Phillips AJL, Kannangara SD, Promputtha I. Fungi vs. Fungi in Biocontrol: An Overview of Fungal Antagonists Applied Against Fungal Plant Pathogens. Front Cell Infect Microbiol. 2020 Nov 30;10:604923. doi: 10.3389/fcimb.2020.604923. PMID: 33330142; PMCID: PMC7734056.
- Trindade M, Moreira AC, Cardillo E, et al. Gestión y prevención de la enfermedad causada por Phytophora cinnamomi en dehesa s y montados. Cicytex [Internet]. 2022. Available from: 978-84-09-20350-5
- Rodríguez-Romero M, Cardillo E, Santiago R, Pulido F. Susceptibility to Phytophthora cinnamomi of six holm oak ( Quercus ilex) provenances: are results under controlled vs. natural conditions consistent? Forest Systems [Internet]. 2022 Jun 13;31(2): e011. Available from: https://doi.org/10.5424/fs/2022312-17977
- Cuenca, B. Mejora de alcornoques y encinas de extremadura ante Phytophthora cinnamomi : selección de genotipos resistentes. In: Gestión del monte: servicios ambientales y bioeconomía: 7th Congreso forestal español;26-30 Jun 2017; Plasencia, Cáceres Extremadura.
- Schwinn FJ. New developments in chemical control of phytophthota. In Phytophthora: Its bioloy, Taxonomy, Ecology and Pathology. St, Paul: American Phytopathological Society;1983. p.327-334
- Coffey MD. Strategies for integrated control of soil borne Phytophthora species. In: Phytophthora. Lucas J.A., Shattock R.C., Shaw D.S., Cooke L.Rl (eds.). Cambridge Univ. Press, Cambridge. 1991; pp. 411-432.
- Sena KL, Crocker E, Vincelli P, Barton CD. Phytophthora cinnamomi as a driver of forest change: Implications for conservation and management. Forest Ecology and Management [Internet]. 2018 Feb 1;409:799–807. Available from: https://doi.org/10.1016/j.foreco.2017.12.022
- Dobrowolski MP, Shearer BL, Colquhoun IJ, O’Brien PA, St J Hardy GE. Selection for decreased sensitivity to phosphite in Phytophthora cinnamomi with prolonged use of fungicide. Plant Pathology [Internet]. 2008 Sep 19;57(5):928-36. Available from: https://doi.org/10.1111/j.1365-3059.2008.01883.x
- Lawrence SA, Robinson HF, Furkert DP, Brimble MA, Gerth ML. Screening a Natural Product-Inspired Library for Anti-Phytophthora Activities. Molecules. 2021 Mar 24;26(7):1819. doi: 10.3390/molecules26071819. PMID: 33804938; PMCID: PMC8037946.
- Fernández-Rebollo P, Leal-Murillo JS, Hidalgo-Fernández T, Alza-Aramburo J, Carbonero-Muñoz D, Godoy-Cancho B, Rodríguez-Molina C. ¿Mantienen las plantas de mostaza su efectividad frente a Phytophthora cinnamomi una vez que han sido henificadas y deshidratadas? In: Libro de resúmenes: XIX congreso de la sociedad española de fitopatología; Sociedad española de fitopatología, Toledo españa;8-10 Oct 2018.p.134. Available from: https://sef.es/sites/default/files/publications/FITOPATOLOGIA_N2.pdf
- Concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC (Regulation (EC) no 1107/2009 of the european parliament and of the council, 21 october 2009)
- Serrano-Moral MS. Control cultural de la podredumbre radical causada por Phytophthora cinnamomi en dehesa s de encina [tesìs doctoral]. Universidad de cordoba;2012.
- Cardillo E, Acevedo A. Susceptibilidad de plantas del entorno de la dehesa extremeña a Phytophthora cinnamomi . Una revisión bibliográfica. Instituto del Corcho, la Madera y el Carbón Vegetal. Gobierno de Extremadura. Susceptibilidad y resistencia a Phytophthora cinnamomi , 6 pp. Científicas y Tecnológicas de Extremadura (CICYTEX).2013; p62.
- Mateus MC, Neves D, Dacunha B, Laczkó E, Maia C, Teixeira R, et al. Structure, anti-Phytophthora and anti-tumor activities of a nortriterpenoid from the rhizome of Phlomis purpurea (Lamiaceae). Phytochemistry [Internet]. 2016 Nov 1; 131:158–64. Available from: https://doi.org/10.1016/j.phytochem.2016.09.004
- Neves D, Caetano P, Oliveira J, Maia C, Horta M, Sousa N, et al. Anti-Phytophthora cinnamomi activity of Phlomis purpurea plant and root extracts. European Journal of Plant Pathology [Internet]. 2013 Dec 18;138(4):835–46. Available from: https://doi.org/10.1007/s10658-013-0357-6
- Alvarez LA,Pérez-Sierra J, Armengol, García-Jiménez J. Characterization of phytophthora nicotianae isolates causing collar and root rot of lavender and rosemary in spain . Journal of Plant Pathology [Internet]. 2007; 89(2):261-264. Available from https://www.researchgate.net/publication/242760310_Characterization_of_phytophthora_nicotianae_isolates_causing_collar_and_root_rot_of_lavender_and_rosemary_in_SPAIN
- González M, Pérez‐Sierra A, Sánchez ME. Phytophthora oleae, a new root pathogen of wild olives. Plant Pathology [Internet]. 2019 Apr 12;68(5):901–7. Available from: https://doi.org/10.1111/ppa.13024
- de Andrade Lourenço D, Branco I, Choupina A. Phytopathogenic oomycetes: a review focusing on Phytophthora cinnamomi and biotechnological approaches. Mol Biol Rep. 2020 Nov;47(11):9179-9188. doi: 10.1007/s11033-020-05911-8. Epub 2020 Oct 17. PMID: 33068230.
- Bosso L, Scelza R, Varlese R, Meca G, Testa A, Rao MA, Cristinzio G. Assessing the effectiveness of Byssochlamys nivea and Scopulariopsis brumptii in pentachlorophenol removal and biological control of two Phytophthora species. Fungal Biol. 2016 Apr;120(4):645-653. doi: 10.1016/j.funbio.2016.01.004. Epub 2016 Jan 14. PMID: 27020163.
- Méndez-Bravo A, Cortazar-Murillo EM, Guevara-Avendaño E, Ceballos-Luna O, Rodríguez-Haas B, Kiel-Martínez AL, Hernández-Cristóbal O, Guerrero-Analco JA, Reverchon F. Plant growth-promoting rhizobacteria associated with avocado display antagonistic activity against Phytophthora cinnamomi through volatile emissions. PLoS One. 2018 Mar 20;13(3):e0194665. doi: 10.1371/journal.pone.0194665. PMID: 29558512; PMCID: PMC5860777.
- Trzewik A, Maciorowski R, Klocke E, Orlikowska T. The influence of Piriformospora indica on the resistance of two rhododendron cultivars to Phytophthora cinnamomi and P. plurivora. Biological Control [Internet]. 2020 Jan 1; 140:104121. Available from: https://doi.org/10.1016/j.biocontrol.2019.104121
- Woo SL, Hermosa R, Lorito M, Monte E. Trichoderma: a multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat Rev Microbiol. 2023 May;21(5):312-326. doi: 10.1038/s41579-022-00819-5. Epub 2022 Nov 22. PMID: 36414835.
- Ruíz-Gómez FJ, Miguel-Rojas C. Antagonistic Potential of Native Trichoderma spp. against Phytophthora cinnamomi in the Control of Holm Oak Decline in dehesa s Ecosystems. Forests [Internet]. 2021 Jul 17;12(7):945. Available from: https://doi.org/10.3390/f12070945
- Liu Y, He P, Munir S, He P, Wu Y, Asad S, Tang Z, He Y. Phytophthora cinnamomi causing root rot on Rhododendron lapponicum and control it using potential biocontrol agents. J Basic Microbiol. 2022 Aug;62(8):937-947. doi: 10.1002/jobm.202200034. Epub 2022 May 12. PMID: 35554952.
- de Andrade Lourenço D, Branco I, Choupina A. A systematic review about biological control of phytopathogenic Phytophthora cinnamomi . Mol Biol Rep. 2022 Oct;49(10):9947-9962. doi: 10.1007/s11033-022-07547-2. Epub 2022 May 18. PMID: 35585380.
- Ruiz Gómez, FJ, Navarro-Cerrillo, RM, Pérez-de-Luque, A. et al. Evaluación de cambios funcionales y estructurales de comunidades de hongos y oomicetos en el suelo de dehesa s declinadas de encina mediante análisis de metabarcodes. Representante científico. 2019; 9, p. 5315. Available from: https://doi.org/10.1038/s41598-019-41804-y
- Cabrera-Puerto RJ, Ruiz-Gómez FJ, Navarro-Cerrillo RM. Beneficial Microorganisms and Water Stress Influence Quercus ilex Seedlings’ Response to Phytophthora cinnamomi Rands. Forests [Internet]. 2023 April 24;14(5):870. Available from: https://doi.org/10.3390/f14050870
- Geilfus C. Microbial and Plant-Based Biostimulants. In: Springer eBooks [Internet]. 2019. p. 131–43. Available from: https://doi.org/10.1007/978-3-030-23197-2_12
- Franzoni G, Cocetta G, Prinsi B, Ferrante A, Espen L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae [Internet]. 22 de Febr de 2022;8(3):189. Available from: https://doi.org/10.3390/horticulturae8030189
- Rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. (Regulation (EU) 2019/1009 of the european parliament and of the council, 05 june 2019).
- Tkaczyk M, Szmidla H, Sikora K.The use of biostimulants containing Ascophyllum nodosum (L.) Le Jolis algal extract in the cultivation and protection of English oak Quercus robur L. seedlings in forest nurseries. Sylwan [Internet]. 2022;166 (4): 244-252. Available from: https://doi.org/10.26202/sylwan.2022032
- Cardillo E, Abad E, Meyer S. Iberian oak decline caused by Phytophthora cinnamomi : A spatiotemporal analysis incorporating the effect of host heterogeneities at landscape scale. Forest Pathology [Internet]. 2021 Jun 31;51(2). Available from: https://doi.org/10.1111/efp.12667
- Cardillo E, Acevedo A, Perez C. Spatial patterns of holm and cork oak decline in Extremadura, Spain. Phytophthora in Forests and Natural Ecosystems. 2012; 6:48. Available from https://forestphytophthoras.org/sites/default/files/proceedings/IUFRO%202014%20final%2023.8MB.pdf
- Jones JD, Dangl JL. The plant immune system. Nature. 2006 Nov 16;444(7117):323-9. doi: 10.1038/nature05286. PMID: 17108957.
- Mandal SM, Chakraborty D, Dey S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav. 2010 Apr;5(4):359-68. doi: 10.4161/psb.5.4.10871. Epub 2010 Apr 7. PMID: 20400851; PMCID: PMC2958585.
- Dorado FJ, Alías JC, Chaves N, Solla A. Warming Scenarios and Phytophthora cinnamomi Infection in Chestnut (Castanea sativa Mill.). Plants (Basel). 2023 Jan 26;12(3):556. doi: 10.3390/plants12030556. PMID: 36771639; PMCID: PMC9921032.
- Daglia M. Polyphenols as antimicrobial agents. Current Opinion In Biotechnology [Internet]. 1 de abril de 2012;23(2):174-81. Disponible en: https://doi.org/10.1016/j.copbio.2011.08.007
- Moctezuma C, Hammerbacher A, Heil M, Gershenzon J, Méndez-Alonzo R, Oyama K. Specific polyphenols and tannins are associated with defense against insect herbivores in the tropical oak Quercus oleoides. J Chem Ecol. 2014 May;40(5):458-67. doi: 10.1007/s10886-014-0431-3. Epub 2014 May 9. PMID: 24809533.
- Salminen JP, Roslin T, Karonen M, Sinkkonen J, Pihlaja K, Pulkkinen P. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J Chem Ecol. 2004 Sep;30(9):1693-711. doi: 10.1023/b:joec.0000042396.40756.b7. PMID: 15586669.
- Pulido FJ, Gallardo A, Sánchez DM, Marcos GM, Romero MR, Hach AS, et al. Determinantes de la resistencia al estrés biótico en una especie forestal modelo: la encina (« Quercus ilex») en los Parques Nacionales [Internet]. Dialnet. 2019. Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=8352887
- Solla A, Milanović S, Gallardo A, Bueno AC, Corcobado T, Escudero YC, et al. Genetic determination of tannins and herbivore resistance in Quercus ilex. Tree Genetics & Genomes [Internet]. 14 de noviembre de 2016;12(6). Disponible en: https://doi.org/10.1007/s11295-016-1069-9
- Morales M, Munné-Bosch S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019 Jul;180(3):1246-1250. doi: 10.1104/pp.19.00405. PMID: 31253746; PMCID: PMC6752910.
- Escandón M, Castillejo-Sánchez MA, Jorrín‐Novo JV, Rey M. Molecular Research on Stress Responses in Quercus spp.: From Classical Biochemistry to Systems Biology through Omics Analysis. Forests [Internet]. 19 de marzo de 2021;12(3):364. Disponible en: https://doi.org/10.3390/f12030364
- Ghanbary E, Kouchaksaraei MT, Zarafshar M, Bader KM, Mirabolfathy M, Ziaei M. Differential physiological and biochemical responses of Quercus infectoria and Q. libani to drought and charcoal disease. Physiologia Plantarum [Internet]. 11 de noviembre de 2019;168(4):876-92. Disponible en: https://doi.org/10.1111/ppl.13027
- Morcillo M, Sales E, Ponce L, Guillén A, Segura J, Arrillaga I. Effect of elicitors on holm oak somatic embryo development and efficacy inducing tolerance to Phytophthora cinnamomi. Sci Rep. 2020 Sep 16;10(1):15166. doi: 10.1038/s41598-020-71985-w. PMID: 32938968; PMCID: PMC7495464.
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006 Nov;28(11):1091-101. doi: 10.1002/bies.20493. PMID: 17041898.
- Huang H, Ullah F, Zhou DX, Yi M, Zhao Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front Plant Sci. 2019 Jun 25;10:800. doi: 10.3389/fpls.2019.00800. PMID: 31293607; PMCID: PMC6603150.
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010 Sep;30(3):161-75. doi: 10.3109/07388550903524243. PMID: 20214435.
- Xiong S, Wang Y, Chen Y, Gao M, Zhao Y, Wu L. Effects of Drought Stress and Rehydration on Physiological and Biochemical Properties of Four Oak Species in China. Plants (Basel). 2022 Mar 2;11(5):679. doi: 10.3390/plants11050679. PMID: 35270149; PMCID: PMC8912384.
- Mohammadi MA, Han X, Zhang Z, Xi Y, Boorboori M, Wang-Pruski G. Phosphite Application Alleviates Pythophthora infestans by Modulation of Photosynthetic and Physio-Biochemical Metabolites in Potato Leaves. Pathogens. 2020 Feb 28;9(3):170. doi: 10.3390/pathogens9030170. PMID: 32121090; PMCID: PMC7157663.
- Guerrero-Sánchez VM, Castillejo MÁ, López-Hidalgo C, Alconada AMM, Jorrín-Novo JV, Rey MD. Changes in the transcript and protein profiles of Quercus ilex seedlings in response to drought stress. J Proteomics. 2021 Jul 15;243:104263. doi: 10.1016/j.jprot.2021.104263. Epub 2021 May 15. PMID: 34000457.
- Coelho AC, Pires R, Schütz G, Santa C, Manadas B, Pinto PIS. Disclosing proteins in the leaves of cork oak plants associated with the immune response to Phytophthora cinnamomi inoculation in the roots: A long-term proteomics approach. PLOS ONE [Internet]. 22 de enero de 2021;16(1):e0245148. Disponible en: https://doi.org/10.1371/journal.pone.0245148
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
