Effect of Ocean Acidification on the Communications among Invertebrates Mediated by Plant-Produced Volatile Organic Compounds
1Functional and Evolutionary Ecology Laboratory, Stazione Zoological Anton Dohrn. Integrative Marine Ecology Department. Benthic Ecology Centre. Villa Dohrn. Punta San Pietro. 80077, Ischia. Italy
2Cologne Biocenter, Workgroup Aquatic Chemical Ecology, University of Cologne, Zülpicher Straße 47b, 50674 Cologne, Germany
Author and article information
Cite this as
Zupo V, Mutalipassi M, Fink P, Di Natale M (2016) Effect of Ocean Acidification on the Communications among Invertebrates Mediated by Plant-Produced Volatile Organic Compounds. Glob J Ecol. 2016; 1(1): 012-018. Available from: 10.17352/gje.000002
Copyright License
© 2016 Zupo V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Chemical communications among plant and animal components are fundamental elements for the functioning and the connectivity of ecosystems. In particular, wound-activated infochemicals trigger specific reactions of invertebrates according to evolutionary constraints, permitting them to identify reycues, escape predators and optimize their behaviors according to specific life strategies. Thus, the orrect flux of information made possible by the production of plant infochemicals and its recognition by given invertebrates is fundamental to assure an appropriate functioning of complex ecosystems. However, global warming and ocean acidification (OA) are deeply influencing the metabolism of organisms and confounding their chemical communications. The production of plant secondary metabolites is influenced by global environmental changes and the OA can modify the effect of infochemicals, inducing dramatic modifications in the behavior of various animals. This research takes into account the effect of volatile organic compounds produced by epiphytes growing on a seagrass and the changes induced by OA in the chemotactic reactions exhibited by associated invertebrates. Our results demonstrate that behavioural influences may hamper the survival of key species of invertebrates, besides the direct effects of OA on their physiology.
OA: Ocean Acidification; VOC(s): Volatile Organic Compound(s)
Introduction
The pH of oceans decreased in the last decades due to high CO2 emissions characterizing the industrial era [1] and a further decrease, in the order of 0.5 points, is forecasted for the next century [2]. The Ocean Acidification (OA) has evident physiologic effects on organisms equipped with external calcareous structures (e.g., coralline algae, crustaceans, corals) and this triggers immediate deleterious effects on their survival and fitness [3]. For example, a reduction of 20-40% in the calcification rates of corals has been recorded since 1880 and a further reduction is forecasted for the year 2062 [4]. Therefore, dramatic ecological changes are forecasted for the next century according to the increases of CO2 levels in the atmosphere, trending to 520 ppm [5].
Besides the direct effects of OA on the physiology of plants and invertebrates, other consequences may be forecasted. In fact, animals use chemical cues to communicate among them and receive infochemicals produced by plants, interpreting their meaning to identify trophic resources or detect the presence of predators [6]. Infochemicals are widely diffused in the aquatic environment [7] and our knowledge of their role and importance is still in its infancy, although several researches demonstrated their importance in aerial environments [8].
The term “infochemical” [9] refers to compounds bearing information that can be received by various species living in the same environment and triggers specific reactions [10]. Volatile organic compounds (VOCs) are among the most interesting infochemicals, because they are quickly transferred to other organisms, even at a long distance from the source of production [11]. Several defence compounds [12] are produced by plants when their tissues are wounded by grazers and they can produce various effects, e.g., they can be toxic for the consumers or their progenies [13], they can produce irritation or avoidance [14], and they can indicate the presence of toxic activity [8]. These effects are quite important even to stabilize the plant and animal communities associated to complex ecosystems, as seagrasses [8]. In fact, wound-activated infochemicals [14] are readily recognized by invertebrates living in the same environments, while they are not recognized by alien species and even by species living in the surrounding environments. Thus, invertebrates strictly associated to the same community recognize toxic or dangerous algae and do not feed on them, while alien invaders may not recognize the danger and feed on toxic algae.
These complex relationships, whose importance has been revealed only in the last decades, are all influenced by OA, because compounds playing an infochemical role may a) be produced differently, b) change their molecular structure or c) become not detectable by animal receiver sensors. Therefore it is important to identify the behavioural effects triggered by plant-produced VOCs upon wounding and study the modification of chemotactic reactions according to the pH of the aqueous medium. To this end we investigated the VOCs produced by algal epiphytes growing on the leaves of Posidonia oceanica, a seagrass exhibiting a primary role for the coastal productivity of the Mediterranean sea [15], and we tested their effects in normal seawater (pH 8.1) and in acidified seawater (pH 7.6), simulating the pH forecasted for the next century, in order to sketch ecological scenarios about the possible effects of OA on the animal communities associated to this fundamental Mediterranean seagrass and the possibility to conserve their actual structure.
Material and Methods
Sampling site and strategy
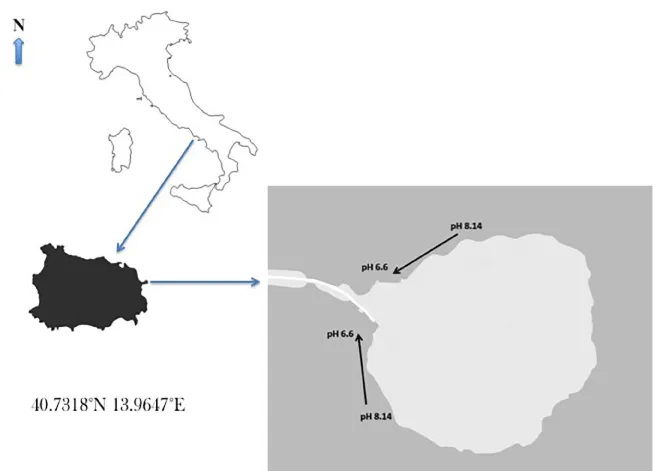
Benthic invertebrates known to be typical of the Posidonia oceanica leaf stratum [16] were collected at two sites off the Castle of Ischia (Bay of Naples). In this area (Figure 1) gaseous CO2 natural emissions [17] produce local acidification over a P. oceanica meadow. A north-south pH gradient is observed along the coastline, from normal seawater pH (8.1) to very acidic levels (6.6). Vagile fauna was sampled using a net driven by scuba divers at an average depth of 5 m, in both acidified and control stations hosting P. oceanica meadows.
Samples were immediately sorted on boat and the main taxa (decapods, molluscs, isopods, amphipods) were separated. The most abundant species present in the samples were identified in the laboratory and separated alive, to test the effects of plant metabolites. Collections performed in both sites were combined in order to obtain generic pools of each species, not biased by their abundance in the normal or in the acidified area. Taking into account the number of individuals sampled, a variety of different taxa and their trophic preferences, 12 species of invertebrates were chosen (Table 1), to be reared in the laboratory and submitted to choice tests for the recognition of VOCs produced by various algae.
Each pool of individuals of a single species was then reared in aerated vessels in a thermostatic chamber (18°C) with a light cycle of 12 hours per day, fed with small portions of epiphytized P. oceanica leaves. When the choice experiments started, animals were starved for 24 hours and slowly adapted to each experimental pH regime, prior to be tested.
Selection and culture of macroalgae and cyanobacteria
Posidonia oceanica leaves were collected in the same site and examined under the stereo-microscope for the presence of epiphytes. Various algal thalli were detached using forceps and moved to sterilized Petri dishes containing f/2 medium. Each 5 days the thalli were examined for their growth, other epiphytes grown on their surface were removed, and they were transferred to fresh f/2 medium. After three months two algae appeared clean and they were grown in axenic conditions. The chosen producers of VOCs were the green alga Enteromorpha prolifera and the red alga Colaconema daviesi. In addition, we isolated thalli of cyanobacteria of the Phormidium group, known for producing various toxic metabolites [18]. We decided to test these three very different epiphytes at two concentrations, to highlight any influence of the medium pH on the chemotactic reactions of invertebrates exhibited as an answer to the VOCs produced by epiphytes after wounding.
Extraction of odours and preparation of agarose gels
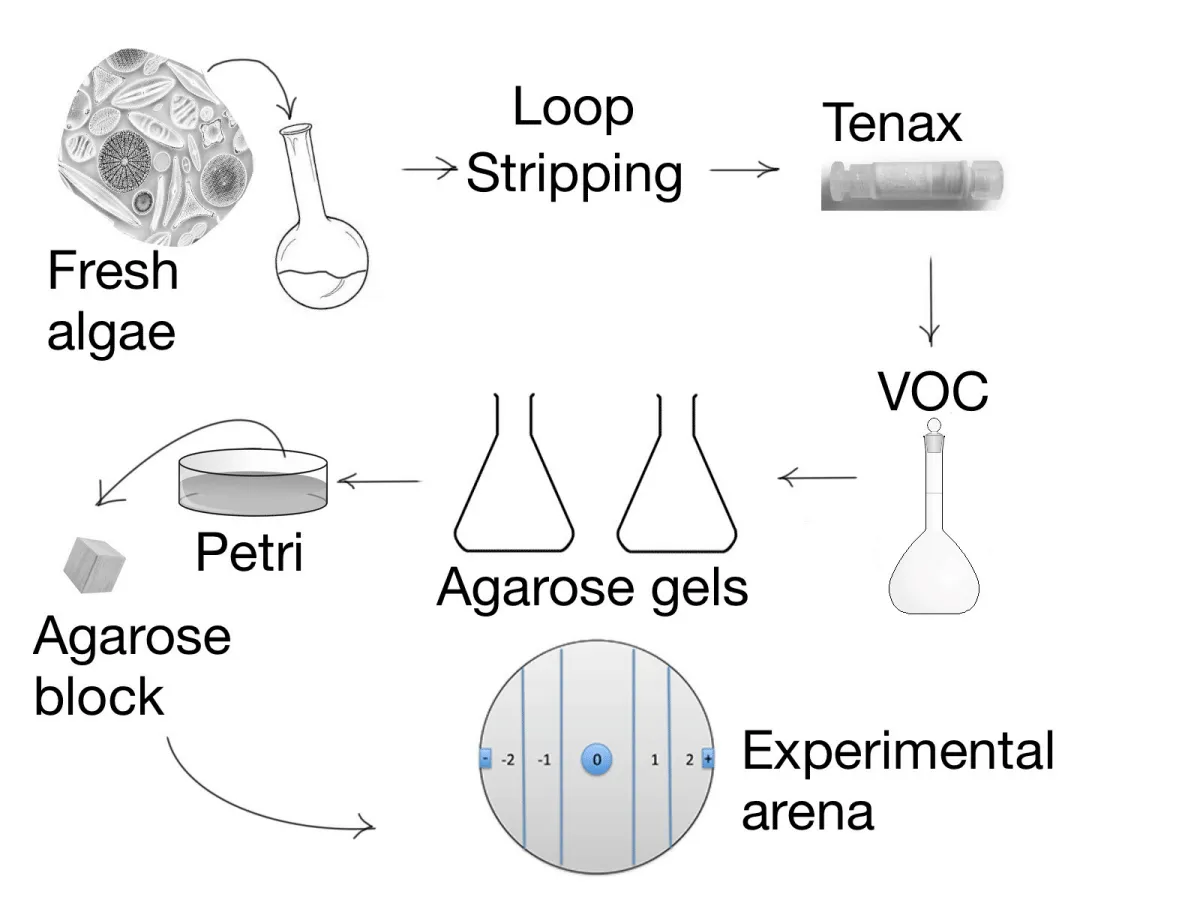
To simulate wounding and production of odours, frozen algal pellets (500 mg FW for each alga) were transferred into 100 ml flasks and activated by thawing them in 25% NaCl [19]. The cells of each microalga were physiologically disintegrated and this led to the activation of the lipoxygenase cascade and the production of volatile compounds [14]. The production of volatile compounds was also indicated by the appearance of a rancid odour. This procedure lasted about 10 min and was sufficient to produce VOCs for our tests. VOCs were concentrated by closed-loop stripping [20] for 45 minutes at 22°C, after which they were adsorbed on a Tenax TA cartridge (Figure 2). The Tenax-filled cartridge was removed, and the VOCs eluted with 6 ml of dietyl ether, as described by [19]. Consequently, the eluate was collected in a clean glass tube and the ether was gently evaporated in a stream of pure nitrogen gas (N2, grade 5.0). The residue was re-dissolved in 70 µl of pure ethanol. The ethanol-diluted odours were then dissolved in the Agarose gel in a way that guaranteed two different concentrations.
Control extracts were obtained according to the same procedures but using filtered seawater without algae. These extracts served as control treatments in the bioassays below described. All samples were stored in glass vials at -80°C to minimize the loss of volatiles.
Volatile compounds were included in agarose gel prior to be offered to the animals, according to [21]. Seven gels were prepared, containing three different producers (two macroalgae and a Cyanobacterium of the Phormidium group) at two concentrations, plus a control (Table 2). Control gels were prepared by dissolving 150 mg of low-melt agarose (Sigma A 9045) in 25 mL of filtered and sterilized seawater at 75 °C. Then we added 400 µl of 0.1 M NaOH to adjust the pH to a value of 8.2 and finally 60 µl of control extract.
The two VOC concentrations, with a higher one that is ten times the first one, simulate the wounding of a fragment of algae by a small grazer (about 10 mm2 of leaf surface area covered with algae) and a large fish (about 100 mm2), respectively, in order to expose our test invertebrates to concentrations having an ecological meaning.
For experimental treatments, 6 or 60 µl (Table 2) of the VOC bouquet obtained as reported in the previous section were added into two beakers containing the agarose solution while it was still liquid but sufficiently cold. The agarose solution added with VOCs was poured into a Petri dish and gelled into a refrigerator (5 °C) prior to cutting the gel into blocks of 1 cm3.
Tests were performed at two odour concentrations as above reported. After cutting 1 cm3 blocks, using a clean glass coverslip (to avoid the addition of metal odours), an odour-containing block of agarose was added to one edge of each experimental arena (+) and a block of control agarose was added to the other edge (-), as indicated in the following section.
Tests on invertebrates
To test the effect of the plant bouquets of odours on various species of benthic invertebrates we conducted odour-choice tests using the technique reported in [21]. Odour choice tests were conducted in order to distinguish positive and negative chemotaxis, mediated by the VOCs of each epiphyte species. Bioassays were performed in 14 cm (diameter) Petri dishes containing 200 ml of seawater (approx. 1 cm water depth), in a climatic chamber at 18°C, with diffused light. Some invertebrates are phototactic and performing the experiment in a low-light environment, with soft and well-diffused light, helped to minimize experimental bias due to phototactic attitudes.
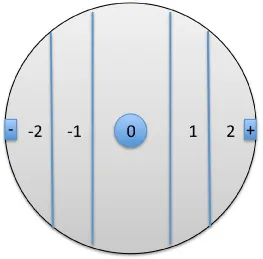
Each arena contained: (i) two rectangles at the two opposite ends of a diameter, one for the VOC sample, the second for the control; (ii) a central circle, used for the initial deployment of 7 individuals of each species, in four replicates; (iii) four vertical lines, delimiting 5 effect zones. The central zone was ranked zero (Figure 3). The two zones near the sample (+) were ranked +1 and +2, respectively. The two zones near the control (-) were ranked -1 and -2, respectively (Figure 3). The positions of the two (+) targets, in every two arenas, were opposed. Thus, such external factors as those that might influence the movements of animals (e.g., light) were kept random between the replicates, thereby excluding directional effects introduced by the experimental setup.
After the start of the experiment, with the deployment of 7 individuals, their movements were recorder each 5 minutes, by checking the number of individuals in each sector for three times. The experiment was completed after 15 minutes.
Statistical analyses
The data collected were organized in matrices of frequency and the significance of differences observed in each sector, for each experiment and in each condition, was tested by means of Student-t at two levels of significance (0.05 and 0.01). In particular, the mean number (±SD) of individuals present in each sector was calculated for each species of grazers vs. each species of epiphytes according to time intervals (0 to 15 min).
The significance of differences in the distribution of individuals between the control areas (-2, -1) and the VOC areas (+2, +1) for each species in the three time intervals were tested by Student-t using GraphPad Prism 4 (GraphPad software). In addition, we used the index proposed by [21] to evaluate the preferences of invertebrates at the two concentrations. This index summarizes the data obtained in positive and negative sectors, yielding a score between -4 (total repulsion) and +4 (total attraction), passing through zero (scarce or null activity of the VOC). For each treatment, we considered the number of individuals in each sector during the experiment. Thereby, a matrix was filled, which contained 5 sectors (from -2 to +2) and 14 treatments (3 epiphyte species x 2 concentrations x 2 pH, plus a control at both pH).
Results
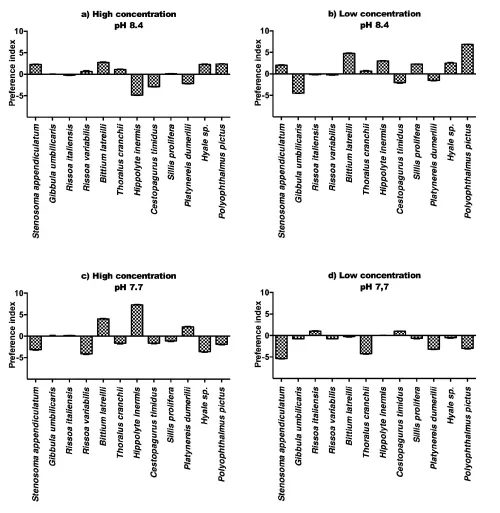
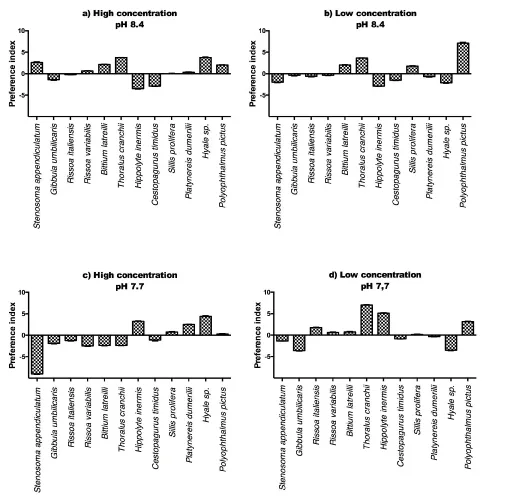
A complex pattern of chemotactic reactions was shown by the test invertebrates at different conditions of pH and according to the considered algae. Enteromorpha prolifera, at normal pH, produced repulsion of the two decapods Hippolyte inermis and Cestopagurus timidus, at high concentration (Figure 4 a) and a slight attraction of the amphipod Hyale sp. and of the polychaete Polyophthalmus pictus. At low concentration the green alga produced attraction of the mollusk Bittium latreilli (Figure 4 b), as well as H. inermis, the polychaete Sillis prolifera, Hyale sp. and Polyophthalmus pictus. Several chemotactic reactions were inverted at low pH (7.7). In fact, at high concentration (Figure 4 c) H. inermis was attracted, while Hyale sp. and Polyophthalmus pictus were repelled. Similarly, at low concentration (Figure 4 d) Stenosoma appendiculatum (slightly attracted at normal pH) was repelled along with Thoralus cranchii and Polyophthalmus pictus.
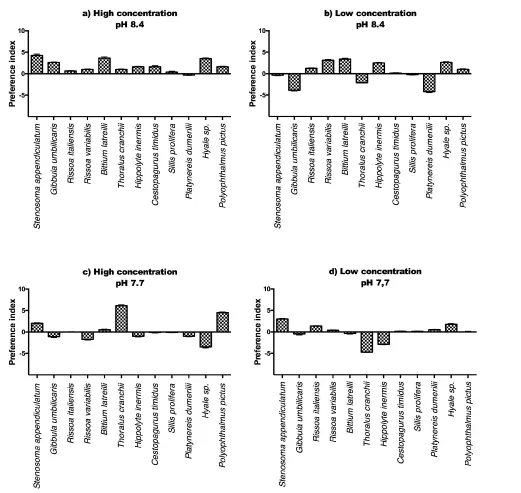
Colaconema daviesi produced a less marked pattern of reactions, since most invertebrates did not exhibit a strong chemotactic reaction when exposed to the VOCs of this red alga. However, it is interesting to observe at normal pH and high concentration (Figure 5 a) Stenosoma appendiculatum exhibited a slight attraction, while the same species, at lower pH, exhibited a clear repulsion (Figure 5 c). Similarly, H. inermis and Thoralus cranchii inverted their reactions of attraction/repulsion at both pH levels, at high concentration (Figure 5 a, c). At low concentration the pattern of reactions for most species was stable at both pH, besides the case of H. inermis, attracted at low pH and repelled at normal pH by this alga.
Cyanobacteria produced consistently a slight attraction of all invertebrates at normal pH and high concentration (Figure 6 a), while a certain degree of repulsion was exhibited at low concentration by the mollusk Gibbula umbilicaris and the polychaete Platynereis dumerilii (Figure 6 b). Most species did not produce any chemotactic reaction at low pH (Figure 6 c, d) but T. cranchii and Polyophthalmus pictus were attracted at high concentration, while the two decapods, H. inermis and T. cranchii, were repelled at the lower concentration.
This complex pattern of reactions has been summarized in Table 3, where the main effects observed are indicated. On the whole, cyanobacteria are scarcely effective on most invertebrates but in some of them (mainly S. appendiculatum, the two decapods, Hyale sp. and P. pictus) they produce attraction at pH 8.4 at high concentration. The pattern is partially repeated at lower pH, with the exception of Hyale sp., inverting its reactions at high concentration and low pH.
In the case of Enteromorpha prolifera, a clear attraction was exhibited by B. latreilli at both pH and all concentrations. In contrast, such species as S. appendiculatum, Hyale sp. and P. pictus clearly inverted their reactions at low pH, since they were attracted at both concentration at normal pH and repelled at pH 7.7.
Finally, Colaconema daviesii produced a clear attraction of B. latreilli, T. cranchii and P. pictus at normal pH but they were repelled at low pH. The reactions were also inverted in the case of H. inermis, repelled by this alga at normal pH but attracted at low pH. Hyale sp. showed a consistent answer at both pH, being attracted at high concentration but repelled by low concentrations of the algal VOCs.
Discussion
It is evident that chemical cues may influence the ecology and the survival of invertebrates associated to aquatic environments [22]. Although chemical cues are especially known for the need of consumers to identify food sources, several recent researches demonstrate that these factors are fundamental to help the avoidance of predators and the recognition of hazards [8]. Several studies indicated the role of diatoms in the production of volatile compounds able to trigger specific reactions by aquatic invertebrates [21] and this research represents the first attempt to explore the ability of macroalgae and cyanobacteria to produce secondary metabolites playing the role of infochemicals for associated invertebrates.
Our results indicate that both algae, as well as cyanobacteria, produce compounds exhibiting a specific activity, although the reactions of invertebrates may change according to the doses offered. This is a common observation in behavioural studies on infochemicals. In fact the same bouquet of compounds may trigger opposite reactions according to the concentration and it is quite difficult to find the correct dose to simulate natural phenomena, since the effect is modulated by the wounded biomass, the proximity to the source of the “odour”, the waves and current speed and other physical factors. Our attempts to simulate and simplify the natural dispersion [23] is only an exercise that provides us with hypotheses to be further explored and tested [21]. In fact, the reactions of various species were different, according to their physiology and their life strategies. Thus, the same infochemical may bring contrasting information to different species according to their trophic and ecological needs [24]. The green alga here tested produced the most evident reaction of attraction, especially in the case of possible grazers. We know that some polychaetes, for example, actively feed on it [25] having a typical herbivore diet. This alga is evidently a possible food for several herbivores and we know that it does not contain large amounts of deterrent compounds [26]. For this reason this alga mostly produced attraction in consumers that might be interested in harvesting it on the leaf stratum and the inversion of their reactions could lead to starvation or to drastic changes of the dietetic patterns of some species, when the pH of the oceans will further decrease.
In contrast, the red alga considered in this study could contain repellent and toxic compounds as a defence against grazers. In fact, in normal conditions it mainly triggers repellence towards several species. It is interesting to observe that in various cases the reactions of the animals were inverted at low pH. Such a shift will produce, in an acidified sea, the feeding on “bad” foods, inducing negative physiologic reactions, since these invertebrates developed the ability to avoid the toxic algal prey and they will loose it in a short period (less than one century), according to the present trends of acidification. Therefore, the effect of the acidification, in the case of the red alga, could result in a loss of the ability, exhibited by several invertebrates, to recognize and avoid bad foods.
In the case of Cyanobacteria this phenomenon is further amplified. It was demonstrated that these bacteria produce sets of toxic compounds able to kill protozoans and large metazoans [18], also shown by toxicity tests on sea urchin embryos [8] and therefore they should be naturally avoided by grazers, if they are unable to detoxify them. Actually, several cyanobacteria are scarcely recognized by invertebrates even at normal pH and this evidence explains their deleterious effects on various animal consumers. However, in acidified conditions several invertebrate species do not show any repellence or even they appear attracted by these possible prey. Therefore, their survival in the leaf stratum of P. oceanica could be hampered by the “wrong” reactions exhibited in acidified conditions.
In conclusion, the present investigation demonstrates that macroalgae and cyanobacteria produce volatile secondary metabolites playing the role of infochemicals for several invertebrates associated to the leaf stratum of P. oceanica. The ecological relationships here described seem comparable to those previously observed with benthic and planktonic diatoms. However, we also demonstrated that the invertebrate reactions might be shifted and inverted in acidified conditions. Therefore, the “wrong” reactions of invertebrates, exhibited at lower pH as an answer to the production of volatile compounds by macroalgae and cyanobacteria, will produce indirect damages to the animal communities associated to P. oceanica ecosystems, as it was demonstrated [27] also for benthic diatoms and the tissues of the plant itself.
The comparison of P. oceanica associated communities at normal pH [16] with the communities stabilized at lower pH [28] indicates clear changes in the patterns of abundance of epiphytes and invertebrates, and our data demonstrate that not only the direct effects of acidification, but also the disturbance of chemical communications [29], may explain the dramatic changes forecasted for the oceans of the next century.
We are grateful to Cpt. V. Rando for his assistance on board the vessel Phoenicia during the sampling operations. MC Gambi, MB Scipione, FP Patti, and M Lorenti identified the species of invertebrates used in our choice experiments. MC Buia and L. Porzio identified the species of algae used for the extraction of VOCs. A. Massa-Gallucci and E. Butera helped the recording of experimental data during the choice experiments.
- Diaz-Pulido G, Cornwall C, Gartrell P, Hurd C, Tran DV (2016) Strategies of dissolved inorganic carbon use in macroalgae across a gradient of terrestrial influence: implications for the Great Barrier Reef in the context of ocean acidification. Coral Reefs 35: 1327-1341. Link: https://goo.gl/CV4Nty
- Turley C, Blackford J, Widdicombe S, Lowe D, Nightingale PD, et al. (2006) Reviewing the impact of increased atmospheric CO 2 on oceanic pH and the marine ecosystem. Avoid Danger Clim Chang 8: 65–70. Link: https://goo.gl/ANCqCC
- Rost B, Zondervan I, Wolf-Gladrow D (2008) Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser 373: 227–237. Link: https://goo.gl/0WcnCR
- Ober GT, Diaz-Pulido G, Thornber C (2016) Ocean acidification influences the biomass and diversity of reef-associated turf algal communities. Mar Biol 163: 204. Link: https://goo.gl/R4hiLZ
- Queiros AM, Huebert KB, Keyl F, Fernandes JA, Stolte W, et al. (2016) Solutions for ecosystem-level protection of ocean systems under climate change. Global Change Biol 22: 3927-3936. Link: https://goo.gl/KgUDfH
- Zupo V, Maibam C (2011) Ecological role of benthic diatoms as regulators of invertebrate physiology and behaviour. In: Compton JC (ed.) Diatoms: Ecology and Life Cycle. Chapter 6: 149–168. Link: https://goo.gl/4XrmRT
- Fink P (2007) Ecological functions of volatile organic compounds in aquatic systems. Mar Freshw Behav Physiol 40: 155–168. Link: https://goo.gl/lBr4yW
- Maibam C, Fink P, Romano G, Buia MC, Gambi MC, et al. (2014) Relevance of wound-activated compounds produced by diatoms as toxins and infochemicals for benthic invertebrates. Mar Biol 161: 1639–1652. Link: https://goo.gl/uXursU
- Dicke M, Sabelis MW (1988) Infochemical terminology: based on cost-benefit analysis rather than origin of compounds? Funct Ecol: 131–139. Link: https://goo.gl/YyTRZ2
- Vos M, Vet LEMM, Wäckers FL, Middelburg JJ, van der Putten WH, et al. (2006) Infochemicals structure marine, terrestrial and freshwater food webs: Implications for ecological informatics. Ecol Inform 1: 23–32. Link: https://goo.gl/UEGnvx
- Selander E, Heuschele J, Nylund GM, Pohnert G, Pavia H, et al. (2016) Solid phase extraction and metabolic profiling of exudates from living copepods. PEERJ 4: 1529. Link: https://goo.gl/I3v2Pp
- Brönmark C, Hansson LA (2012) Aquatic chemical ecology: new directions and challenges for the future. Chem Ecol Aquat Syst Oxford Univ Press Oxford: 272–278. Link: https://goo.gl/v0fY7l
- Hammann M, Rempt M, Pohnert G, Wang G, Boo SM, et al. (2016) Increased potential for wound activated production of Prostaglandin E-2 and related toxic compounds in non-native populations of Gracilaria vermiculophylla. Harmful algae 51: 81-88. Link: https://goo.gl/J0HyM3
- Jüttner F (2005) Evidence that polyunsaturated aldehydes of diatoms are repellents for pelagic crustacean grazers. Aquat Ecol 39: 271–282. Link: https://goo.gl/hrJN3A
- Apostolaki ET, Vizzini S, Hendriks IE, Olsen YS (2014) Seagrass ecosystem response to long-term high CO 2 in a Mediterranean volcanic vent. Marine Environmental Research 99: 9-15. Link: https://goo.gl/u2TMA4
- Gambi MC, Lorenti M, Russo GF, Scipione MB, Zupo V (1992) Depth and seasonal distribution of some groups of the vagile fauna of the Posidonia oceanica leaf stratum: structural and trophic analyses. Mar Ecol 13: 17–39. Link: https://goo.gl/Tegu53
- Cigliano M, Gambi MC, Rodolfo-Metalpa R, Patti FP, Hall-Spencer JM (2010) Effects of ocean acidification on invertebrate settlement at volcanic CO 2 vents. Mar Biol 157: 2489–2502. Link: https://goo.gl/66rSBJ
- Palinska KA, Deventer B, Hariri K, Łotocka M (2011) A taxonomic study on Phormidium–group (cyanobacteria) based on morphology, pigments, RAPD molecular markers and RFLP analysis of the 16S rRNA gene fragment. Fottea 11: 41–55. Link: https://goo.gl/BaamlT
- Fink P, Von Elert E, Jüttner F (2006) Oxylipins from freshwater diatoms act as attractants for a benthic herbivore. Arch für Hydrobiol 167: 561–574. Link: https://goo.gl/S8R9E6
- Shaaban H, Gorecki T (2015) Current trends in green liquid chromatography for the analysis of pharmaceutically active compounds in the environmental water compartments. Talanta 132: 739-752. Link: https://goo.gl/BewsMe
- Jüttner F, Watson SB, Von Elert E, Köster O (2010) β-Cyclocitral, a grazer defence signal unique to the cyanobacterium Microcystis. J Chem Ecol 36: 1387–1397. Link: https://goo.gl/ZB2JnO
- Derby CD, Sorensen PW (2008) Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J Chem Ecol 34: 898–914. Link: https://goo.gl/TFUUw0
- Weissburg M, Atkins L, Berkenkamp K, Mankin D (2012) Dine or dash? Turbulence inhibits blue crab navigation in attractive-aversive odor plumes by altering signal structure encoded by the olfactory pathway. J Exp Biol 215: 4175–4182. Link: https://goo.gl/KeA3c8
- Sbarbati A, Osculati F (2006) Allelochemical communication in vertebrates: kairomones, allomones and synomones. Cells Tissues Organs 183: 206–219. Link: https://goo.gl/hoJy48
- Gambi MC, Musco L, Giangrande A, Badalamenti F, Micheli F, Kroeker KJ (2016) Distribution and functional traits of polychaetes in a CO 2 vent system: winners and losers among closely related species. Mar Ecol Progr Ser 550: 121-134. Link: https://goo.gl/L2zt75 v
- Bravo-Linares CM, Mudge SM, Loyola-Sepulveda RH (2010) Production of volatile organic compounds (VOCs) by temperate macroalgae: The use of solid phase microextraction (SPME) coupled to GC-MS as method of analysis. J Chil Chem Soc 55: 227–232. Link: https://goo.gl/LvSe2C
- Zupo V, Maibam C, Buia MC, Gambi MC, Patti FP, et al. (2015) Chemoreception of the seagrass Posidonia oceanica by benthic invertebrates is altered by seawater acidification. J Chem Ecol 41: 766–779. Link: https://goo.gl/SKVqmu
- Garrard SL, Gambi MC, Scipione MB, Patti MP, Lorenti M, et al. (2014) Indirect effects may buffer negative responses of seagrass invertebrate communities to ocean acidification. J Exp Mar Biol Ecol 461: 31–38. Link: https://goo.gl/0XaAY5
- Roggatz CC, Lorch M, Hardege JD, Benoit DM (2016) Ocean acidification affects marine chemical communication by changing structure and function of peptide signalling molecules. Global Change Biology 22: 3914-3926. Link: https://goo.gl/KnNaSF
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
